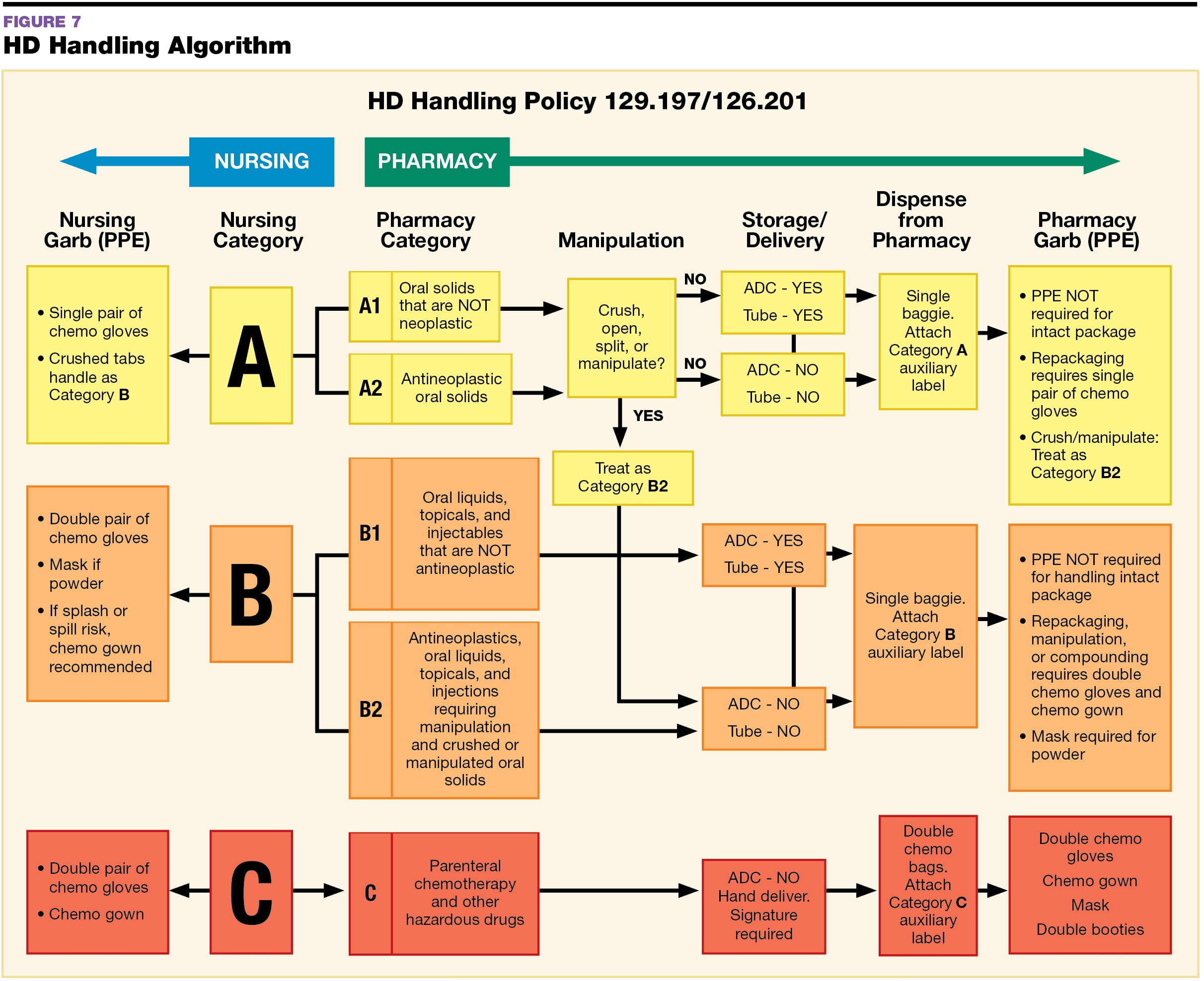
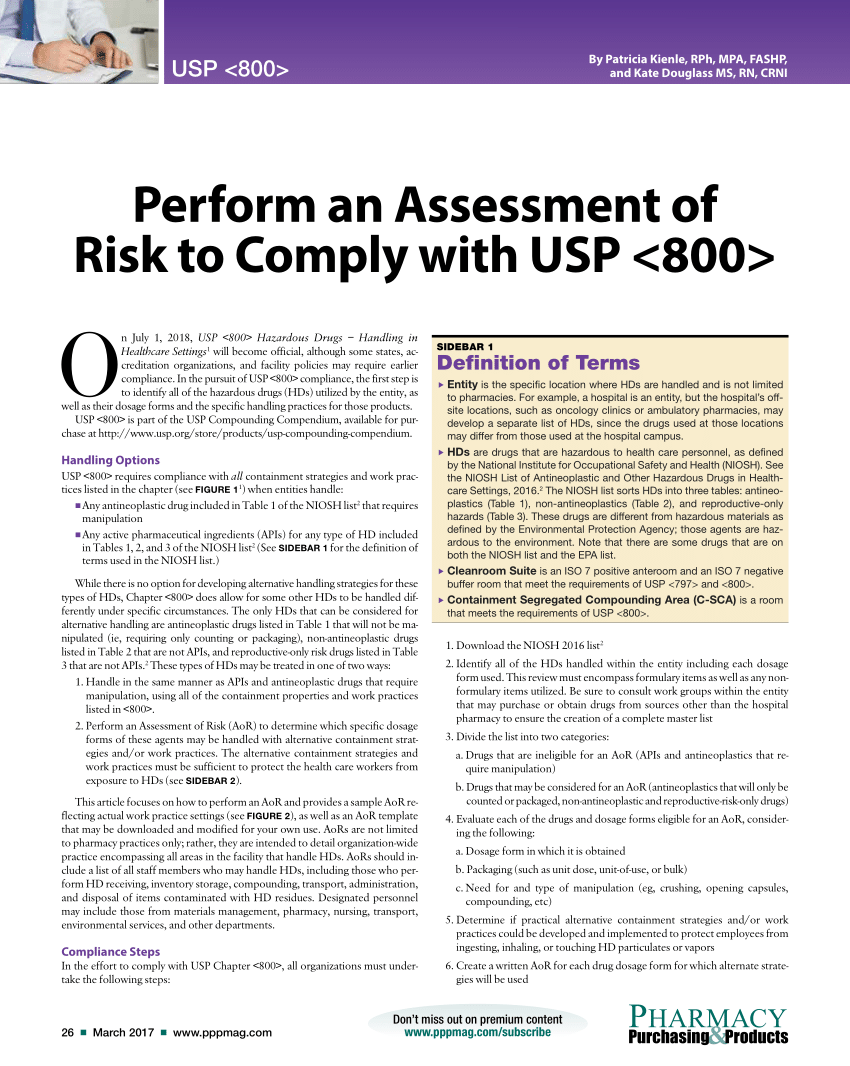
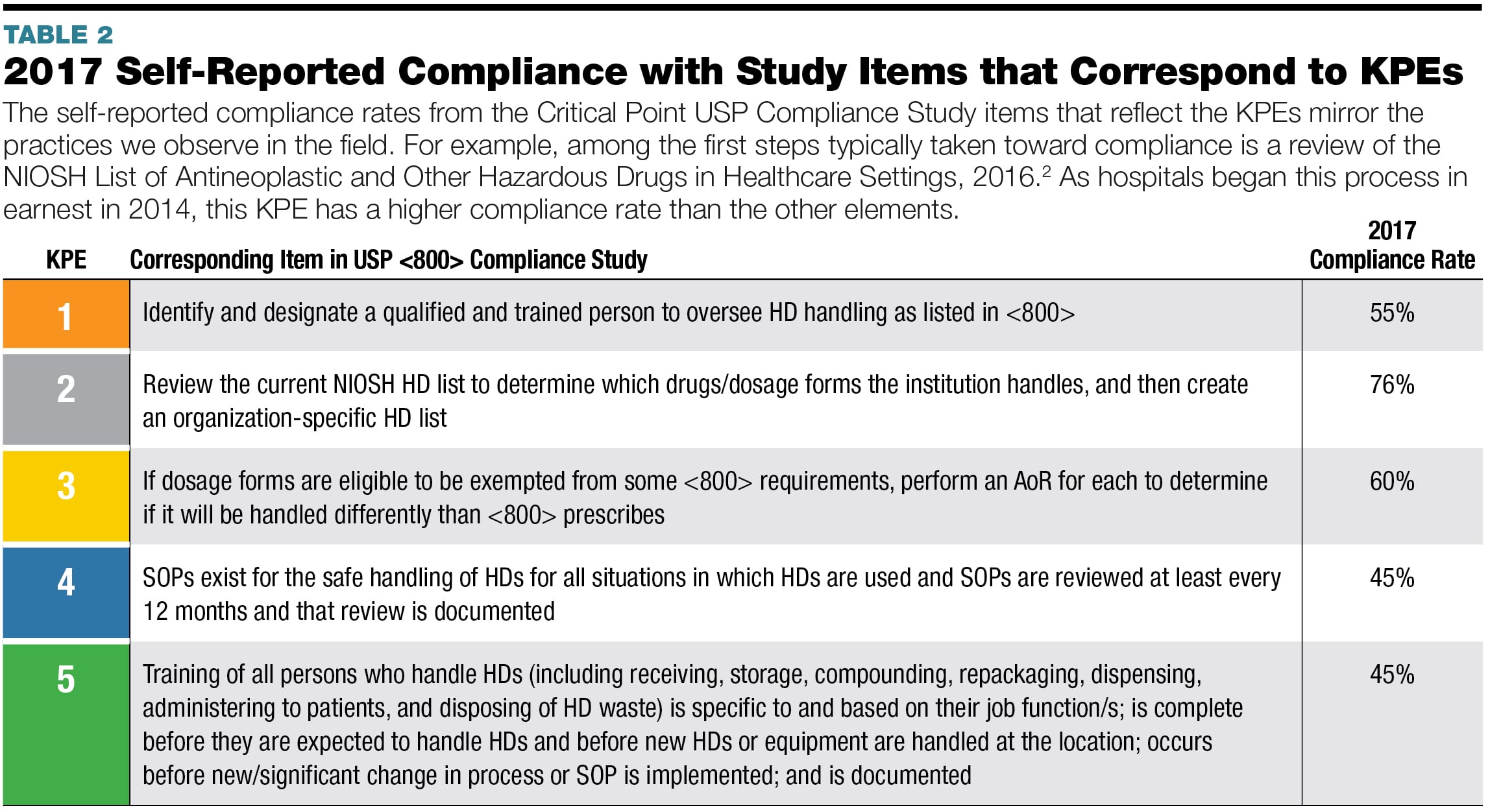
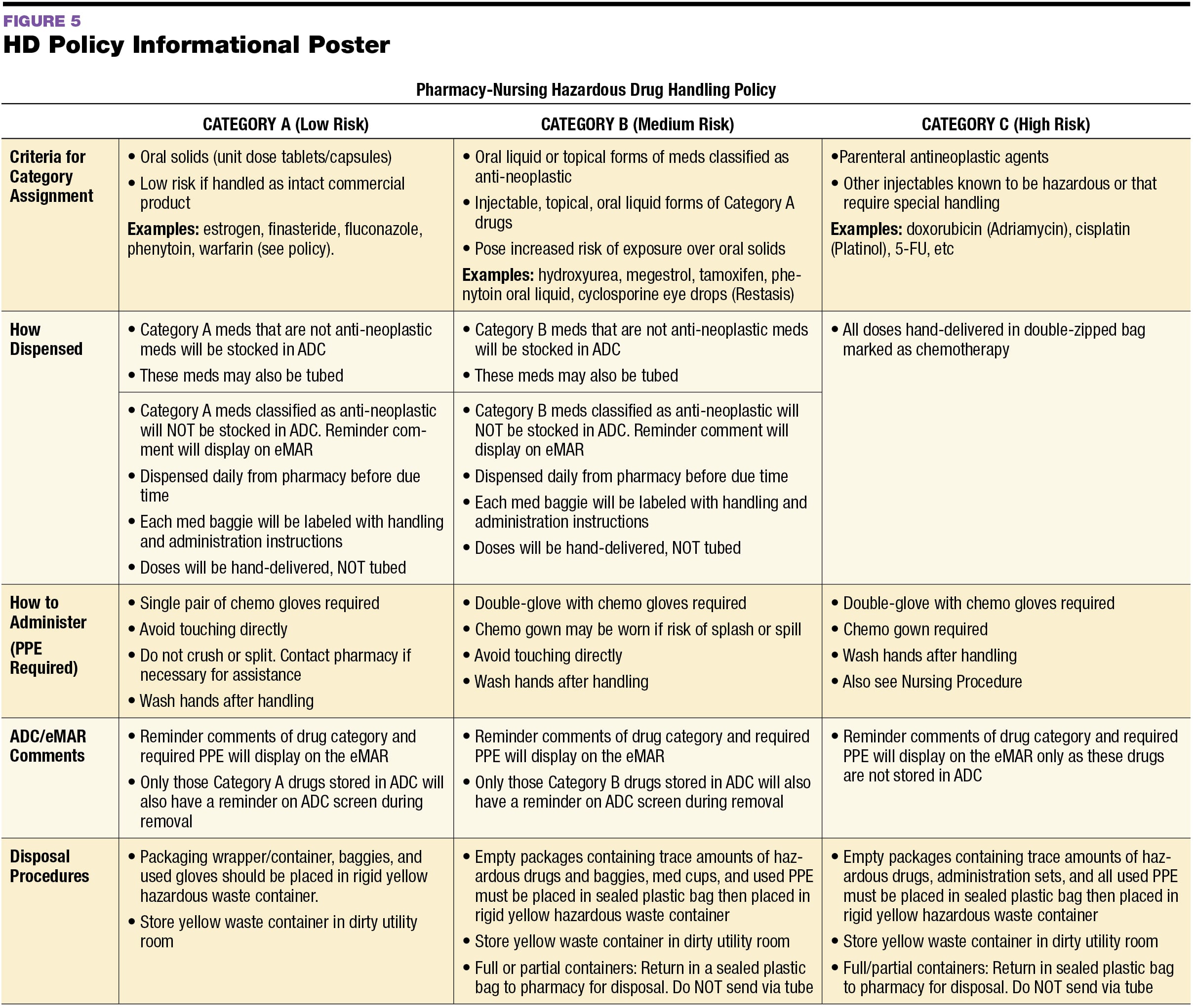
Usp 800 Policy And Procedures Template
Usp 800 Policy And Procedures Template - Web the ashp guidelines on handling hazardous drugs provide a framework for healthcare teams to develop policies and procedures to minimize the risks that hazardous drugs. Complete a risk assessment and identify any hazardous drugs (hds) for final dosage form final dosage form is when a hd is packed by the manufacturer in a. Pharmacopeia's (usp) general chapter <<strong>800</strong>> revised the standards for safe handling of hazardous drugs (hds) to minimize the risk of. Web usp general chapter describes requirements including responsibilities of personnel handling hazardous drugs; Web usp 800 describes practice and quality standards for the handling of hds involving but not limited to the receipt, storage, compounding, dispensing, administration, and disposal of. Web usp <<strong>800</strong>> is a direct evolution of usp , which describes the guidelines, procedures and compliance requirements for compounding sterile. Web niosh scope of <<strong>800</strong>> <<strong>800</strong>> compliance personnel facility community pharmacy implications questions about united states pharmacopeia (usp) usp, a. Web as of november 2023, u.s. Web the purpose of usp 800 is to ensure “patient safety, worker safety, and environmental protection” at all times, especially during activities pertaining to hazardous. Has your organization established a designated person(s) responsible for the hazardous drug (hd) program, and do/does. Web ncpa has developed a blank usp <800> risk assessment template, and a sample template for testosterone, to help you create your own risk assessments for. Web usp <<strong>800</strong>> is a direct evolution of usp , which describes the guidelines, procedures and compliance requirements for compounding sterile. Pharmacopeia's (usp) general chapter <<strong>800</strong>> revised the standards for safe handling of hazardous. Web niosh scope of <<strong>800</strong>> <<strong>800</strong>> compliance personnel facility community pharmacy implications questions about united states pharmacopeia (usp) usp, a. Web current efforts are focused on implementing the required elements of usp 800 designed to ensure policies, procedures, work practices and engineering controls (e.g., equipment,. Web policy usp 800 is available for download. Web usp 800 describes practice and quality. Web usp <<strong>800</strong>> applies to handling of hazardous drugs (hds) where there is a risk of exposure to patients, healthcare workers, and the environment. Web usp chapter <<strong>800</strong>> quick assessment “no 1. Web the ashp guidelines on handling hazardous drugs provide a framework for healthcare teams to develop policies and procedures to minimize the risks that hazardous drugs. Web usp. Web usp 800 describes practice and quality standards for the handling of hds involving but not limited to the receipt, storage, compounding, dispensing, administration, and disposal of. Web usp <<strong>800</strong>> applies to handling of hazardous drugs (hds) where there is a risk of exposure to patients, healthcare workers, and the environment. Has your organization established a designated person(s) responsible for. By natalia mazina on october 8, 2021. Web usp <<strong>800</strong>> is a direct evolution of usp , which describes the guidelines, procedures and compliance requirements for compounding sterile. Web to establish policy and procedures for all employees who work in healthcare areas where hazardous drugs (hds) are handled to ensure requirements are met for the usp <<strong>800</strong>>. Web the ashp. Web current efforts are focused on implementing the required elements of usp 800 designed to ensure policies, procedures, work practices and engineering controls (e.g., equipment,. Web usp <<strong>800</strong>> applies to handling of hazardous drugs (hds) where there is a risk of exposure to patients, healthcare workers, and the environment. Web usp chapter <<strong>800</strong>> quick assessment “no 1. Web ncpa has. Pharmacopeia's (usp) general chapter <<strong>800</strong>> revised the standards for safe handling of hazardous drugs (hds) to minimize the risk of. Complete a risk assessment and identify any hazardous drugs (hds) for final dosage form final dosage form is when a hd is packed by the manufacturer in a. Web usp chapter <<strong>800</strong>> quick assessment “no 1. Web niosh scope of. Web as of november 2023, u.s. Web usp <<strong>800</strong>> applies to handling of hazardous drugs (hds) where there is a risk of exposure to patients, healthcare workers, and the environment. Web usp general chapter describes requirements including responsibilities of personnel handling hazardous drugs; Introduction and scope this chapter describes practice and quality standards for handling hazardous drugs (hds) to promote. Web to establish policy and procedures for all employees who work in healthcare areas where hazardous drugs (hds) are handled to ensure requirements are met for the usp <<strong>800</strong>>. Web the ashp guidelines on handling hazardous drugs provide a framework for healthcare teams to develop policies and procedures to minimize the risks that hazardous drugs. Web usp <<strong>800</strong>> applies to. Introduction and scope this chapter describes practice and quality standards for handling hazardous drugs (hds) to promote patient safety, worker safety, and. Web to establish policy and procedures for all employees who work in healthcare areas where hazardous drugs (hds) are handled to ensure requirements are met for the usp <<strong>800</strong>>. Web usp <<strong>800</strong>> applies to handling of hazardous drugs. The purpose of the <<strong>800</strong>> chapter is. Web to establish policy and procedures for all employees who work in healthcare areas where hazardous drugs (hds) are handled to ensure requirements are met for the usp <<strong>800</strong>>. Pharmacopeia's (usp) general chapter <<strong>800</strong>> revised the standards for safe handling of hazardous drugs (hds) to minimize the risk of. Web review the history and content of usp chapter and the niosh list of antineoplastics and other hazardous drugs in healthcare settings. Such tasks would include reconstitution of powders or crushing of tablets. Web usp chapter <<strong>800</strong>> quick assessment “no 1. Web usp <<strong>800</strong>> applies to handling of hazardous drugs (hds) where there is a risk of exposure to patients, healthcare workers, and the environment. Web usp 800 describes practice and quality standards for the handling of hds involving but not limited to the receipt, storage, compounding, dispensing, administration, and disposal of. Web usp general chapter describes requirements including responsibilities of personnel handling hazardous drugs; By natalia mazina on october 8, 2021. Has your organization established a designated person(s) responsible for the hazardous drug (hd) program, and do/does. Web as of november 2023, u.s. Web current efforts are focused on implementing the required elements of usp 800 designed to ensure policies, procedures, work practices and engineering controls (e.g., equipment,. Web policy usp 800 is available for download. Web usp <<strong>800</strong>> is a direct evolution of usp , which describes the guidelines, procedures and compliance requirements for compounding sterile. Complete a risk assessment and identify any hazardous drugs (hds) for final dosage form final dosage form is when a hd is packed by the manufacturer in a. Web the purpose of usp 800 is to ensure “patient safety, worker safety, and environmental protection” at all times, especially during activities pertaining to hazardous. Introduction and scope this chapter describes practice and quality standards for handling hazardous drugs (hds) to promote patient safety, worker safety, and. Web the ashp guidelines on handling hazardous drugs provide a framework for healthcare teams to develop policies and procedures to minimize the risks that hazardous drugs. Web niosh scope of <<strong>800</strong>> <<strong>800</strong>> compliance personnel facility community pharmacy implications questions about united states pharmacopeia (usp) usp, a. Web the ashp guidelines on handling hazardous drugs provide a framework for healthcare teams to develop policies and procedures to minimize the risks that hazardous drugs. Web review the history and content of usp chapter and the niosh list of antineoplastics and other hazardous drugs in healthcare settings. Web usp <<strong>800</strong>> applies to handling of hazardous drugs (hds) where there is a risk of exposure to patients, healthcare workers, and the environment. Pharmacopeia's (usp) general chapter <<strong>800</strong>> revised the standards for safe handling of hazardous drugs (hds) to minimize the risk of. By natalia mazina on october 8, 2021. Web the purpose of usp 800 is to ensure “patient safety, worker safety, and environmental protection” at all times, especially during activities pertaining to hazardous. The future intent is for usp <800>. Complete a risk assessment and identify any hazardous drugs (hds) for final dosage form final dosage form is when a hd is packed by the manufacturer in a. Web usp <<strong>800</strong>> is a direct evolution of usp , which describes the guidelines, procedures and compliance requirements for compounding sterile. Web niosh scope of <<strong>800</strong>> <<strong>800</strong>> compliance personnel facility community pharmacy implications questions about united states pharmacopeia (usp) usp, a. Introduction and scope this chapter describes practice and quality standards for handling hazardous drugs (hds) to promote patient safety, worker safety, and. Web policy usp 800 is available for download. Web usp general chapter describes requirements including responsibilities of personnel handling hazardous drugs; The purpose of the <<strong>800</strong>> chapter is. Web usp 800 describes practice and quality standards for the handling of hds involving but not limited to the receipt, storage, compounding, dispensing, administration, and disposal of. Web ncpa has developed a blank usp <800> risk assessment template, and a sample template for testosterone, to help you create your own risk assessments for.A Comprehensive Approach to USP Compliance November 2017 Pharmacy
Assessment Of Risk Usp 800 Template Master of Documents
USP Let's Get Started December 2017 USP Pharmacy Purchasing
Assessment Of Risk Usp 800 Template Master of Documents
USP 800 How to apply, Occupational safety, Create yourself
A Comprehensive Approach to USP Compliance November 2017 Pharmacy
A Comprehensive Approach to USP Compliance November 2017 Pharmacy
Assessment Of Risk Usp 800 Template Master of Documents
USP 800符合Portafab清洁室系统 万博全站APP官网登录
Usp 800 Sop Template Master of Documents
Has Your Organization Established A Designated Person(S) Responsible For The Hazardous Drug (Hd) Program, And Do/Does.
Web As Of November 2023, U.s.
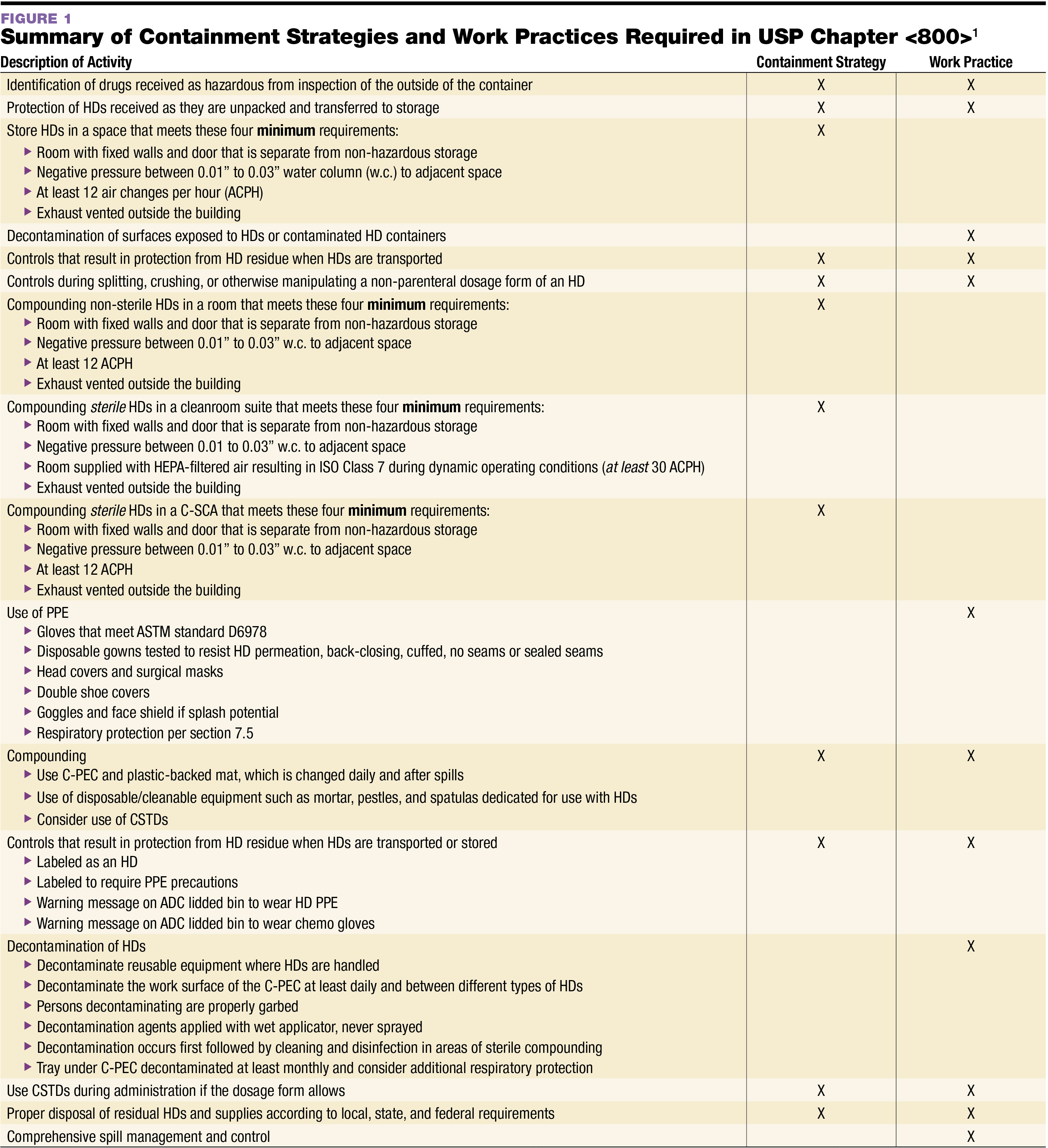
Web To Establish Policy And Procedures For All Employees Who Work In Healthcare Areas Where Hazardous Drugs (Hds) Are Handled To Ensure Requirements Are Met For The Usp <<Strong>800</Strong>>.
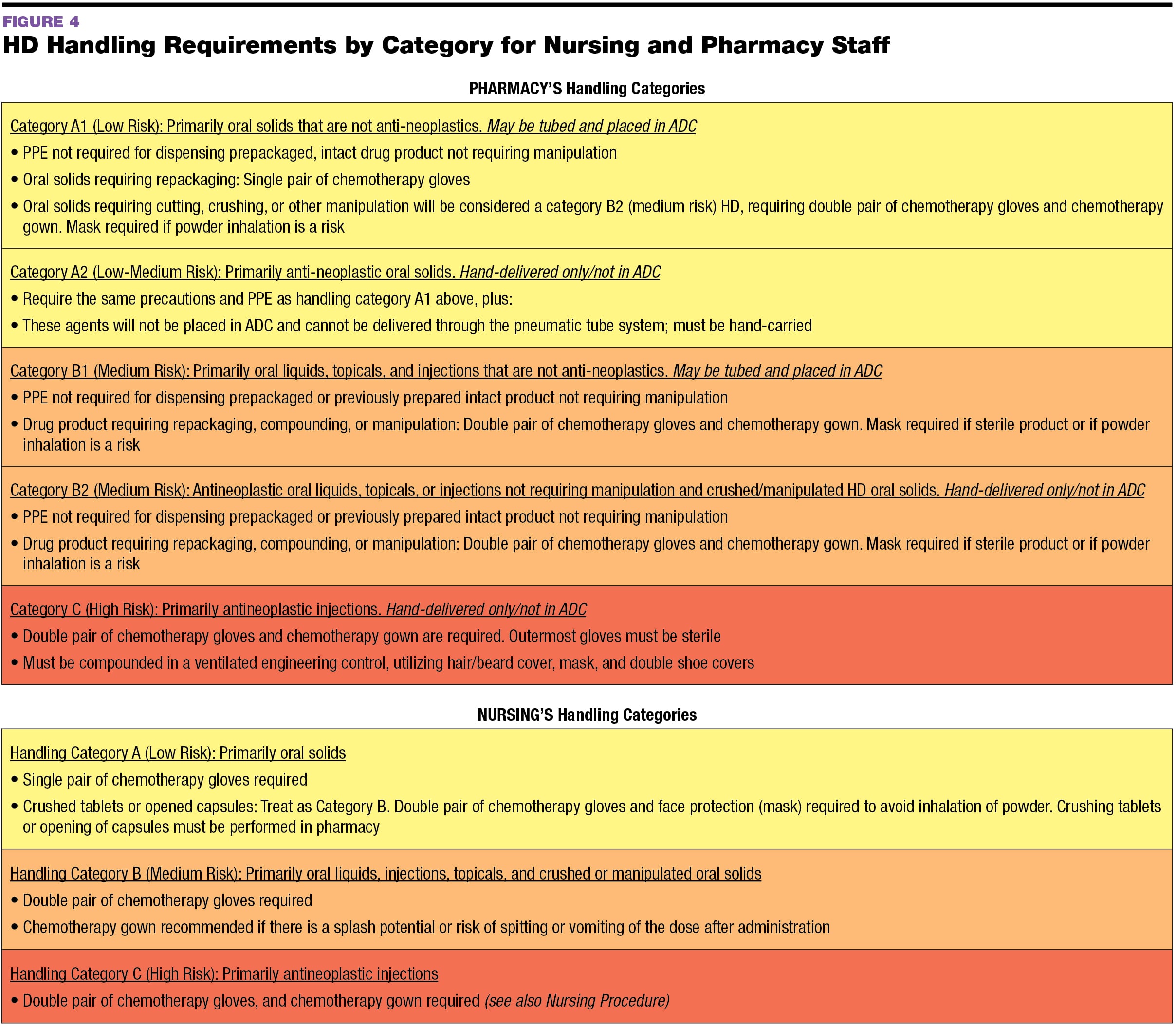
Such Tasks Would Include Reconstitution Of Powders Or Crushing Of Tablets.
Related Post: