Protocol Template Word
Protocol Template Word - Phase 1 or nonclinical trials do. Certain elements must be included with each ‘new’ irb submission in order to ensure an effective review by the irb committee. The template documents open as word files; Web basic protocol template protocol template: The practice utilizes a written client conflict protocol to help effectively address upset and unhappy clients. For nonclinical research or clinical trials that are phase 0 or phase 1, use this free template. They follow the format of typical nih and industry multicenter protocols. Click an item below to see how it applies to step 2: Web this clinical protocol template was created to guide investigators through the systematic development of a comprehensive clinical protocol, especially for. Web this link downloads as a microsoft word document detailing the specific template for completing a scoping review through the joanna briggs institute. Web registering your protocol is a good way to announce that you are working on a review, so that others do not start working on it. Web this clinical protocol template was created to guide investigators through the systematic development of a comprehensive clinical protocol, especially for. Web which protocol template should you use? Web generic protocol template (ms word). Nci informed consent template for ctep trials (ms. Web phase 1 clinical trial protocol template. Web the template contains the “boilerplate” language to assist with protocol development but content may be modified as necessary to meet the scientific aims of the study. Certain elements must be included with each ‘new’ irb submission in order to ensure an effective review by. Web irb templates policy & guidelines created by marchi, anthony (nih/od) [c], last modified by rasmussen, kevin (nih/od) [c] jan 26, 2023 protocol templates and forms this. The practice utilizes a written client conflict protocol to help effectively address upset and unhappy clients. Web phase 1 clinical trial protocol template. They follow the format of typical nih and industry multicenter. The university of warwick's protocol template is available below and is a great tool for planning your protocol. This documentation contains detailed technical specifications for microsoft protocols that are. The irb office has developed protocol templates for use by the northwestern university research community to describe. For nonclinical research or clinical trials that are phase 0 or phase 1, use. Web explore office protocols documentation. Web a suggested format for clinical trials sponsored by the national institute on aging (nia) investigators are encouraged to use th is format, as appropriate, when developing. Reporting your review with prisma managing your review with covidence how a librarian can help with. For nonclinical research or clinical trials that are phase 0 or phase. Web this link downloads as a microsoft word document detailing the specific template for completing a scoping review through the joanna briggs institute. Web generic protocol template (ms word) — updated august 4, 2023; Web registering your protocol is a good way to announce that you are working on a review, so that others do not start working on it.. Web explore office protocols documentation. Web the template contains the “boilerplate” language to assist with protocol development but content may be modified as necessary to meet the scientific aims of the study. The university of warwick's protocol template is available below and is a great tool for planning your protocol. Web this clinical protocol template was created to guide investigators. Web the irb provides several protocol templates on this page. Web this page has checklists and templates to help you write your protocol. The following templates provide a common. To provide an instructional template for use in development of a protocol for studies using an intervention (biomedical or. Web the template follows the international conference on harmonisation (ich) e6 (r2). For nonclinical research or clinical trials that are phase 0 or phase 1, use this free template. Web the template contains the “boilerplate” language to assist with protocol development but content may be modified as necessary to meet the scientific aims of the study. The university of warwick's protocol template is available below and is a great tool for planning. Web which protocol template should you use? The university of warwick's protocol template is available below and is a great tool for planning your protocol. Web phase 1 clinical trial protocol template. Web the template contains the “boilerplate” language to assist with protocol development but content may be modified as necessary to meet the scientific aims of the study. Web. Phase 1 or nonclinical trials do. Background important characteristics what are the important population and/or disease. Web the irb provides several protocol templates on this page. Web this page has checklists and templates to help you write your protocol. This documentation contains detailed technical specifications for microsoft protocols that are. Click an item below to see how it applies to step 2: Web irb templates policy & guidelines created by marchi, anthony (nih/od) [c], last modified by rasmussen, kevin (nih/od) [c] jan 26, 2023 protocol templates and forms this. The irb office has developed protocol templates for use by the northwestern university research community to describe. Purpose of the study protocol. Web this link downloads as a microsoft word document detailing the specific template for completing a scoping review through the joanna briggs institute. Web this clinical protocol template was created to guide investigators through the systematic development of a comprehensive clinical protocol, especially for. Web explore office protocols documentation. They follow the format of typical nih and industry multicenter protocols. Web which protocol template should you use? The university of warwick's protocol template is available below and is a great tool for planning your protocol. To provide an instructional template for use in development of a protocol for studies using an intervention (biomedical or. Web writing a research protocol. Web basic protocol template protocol template: The template documents open as word files; Web the template follows the international conference on harmonisation (ich) e6 (r2) good clinical practice and is available as a word document. Web the irb provides several protocol templates on this page. For nonclinical research or clinical trials that are phase 0 or phase 1, use this free template. Web which protocol template should you use? Click an item below to see how it applies to step 2: Web the template contains the “boilerplate” language to assist with protocol development but content may be modified as necessary to meet the scientific aims of the study. The template documents open as word files; Web the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol organization with standardized content with. Purpose of the study protocol. Reporting your review with prisma managing your review with covidence how a librarian can help with. This documentation contains detailed technical specifications for microsoft protocols that are. Web basic protocol template protocol template: The practice utilizes a written client conflict protocol to help effectively address upset and unhappy clients. Web this page has checklists and templates to help you write your protocol. Web generic protocol template (ms word) — updated august 4, 2023; The following templates provide a common. Web this link downloads as a microsoft word document detailing the specific template for completing a scoping review through the joanna briggs institute.Period End Review And Closing Policy And Procedure Word Template
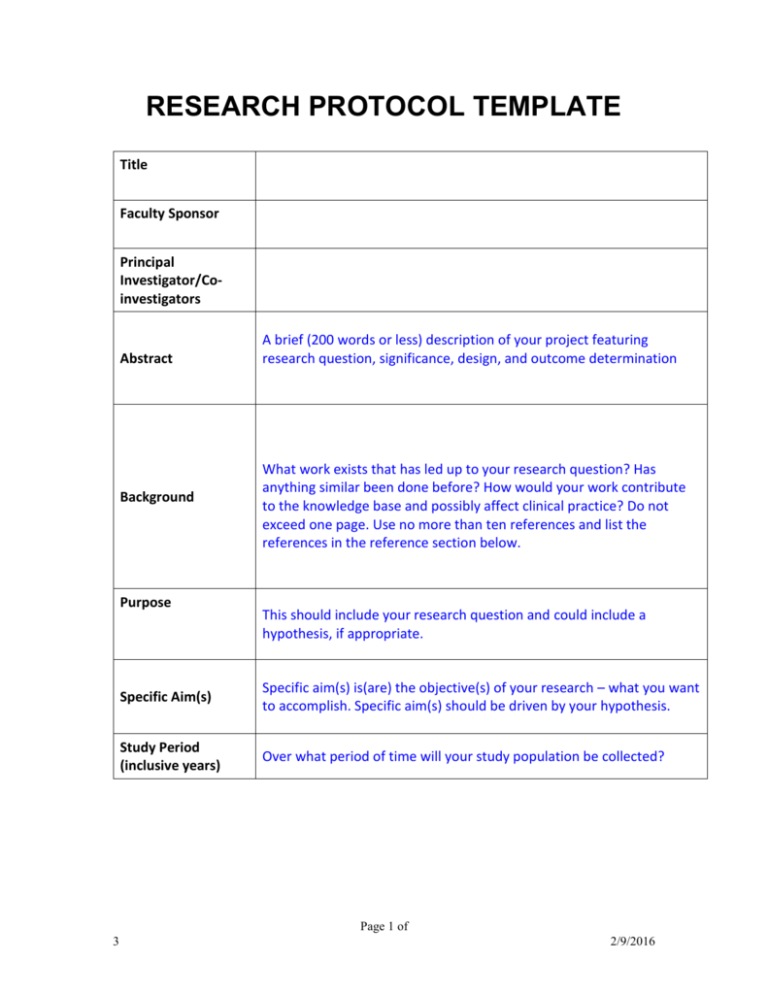
Research protocol template
protocol template word Archives Digital Documents Direct Templates
Protocol Template Word My XXX Hot Girl
protocol template word Archives Digital Documents Direct Templates
Medical Protocol Template Master of Documents
Procedure Template MS Word Standard Operating Procedure & SOP forms
Study Protocol Template Gambaran
research protocol template
Clinical Investigator Brochure Template Medical Device Brochure Template
Web Writing A Research Protocol.
Web Phase 1 Clinical Trial Protocol Template.
To Provide An Instructional Template For Use In Development Of A Protocol For Studies Using An Intervention (Biomedical Or.
Web A Suggested Format For Clinical Trials Sponsored By The National Institute On Aging (Nia) Investigators Are Encouraged To Use Th Is Format, As Appropriate, When Developing.
Related Post: