Phase 1 Clinical Trial Protocol Template
Phase 1 Clinical Trial Protocol Template - Web phase 1 clinical trial protocol template. Web 2.2 phase 1 clinical trial protocol. The clinical trials electronic protocol writing. Web this template aims to facilitate the development of phase 2 and 3 clinical trial protocols. Web the ich m11 clinical electronic structured harmonised protocol. Web the clinical trials electronic protocol writing template/tool provides a. Web 3 rows this protocol template aims to facilitate the development of two. Web phase 1 clinical trial protocol. Web clinical trial protocols should include a clear description of trial design and patient. Web suggested templates for phase 1 and 2 clinical trials. Web guidance documents listed below represent the agency's current thinking. Web 3 rows this protocol template aims to facilitate the development of two. Web phase 1 clinical trial protocol template. Web clinical trial protocols should include a clear description of trial design and patient. Web 2.2 phase 1 clinical trial protocol. Web phase 1 clinical trial protocol template. Web suggested templates for phase 1 and 2 clinical trials. Clinical trial protocol version number: Web if yes, indicate whether this is an applicable clinical trial as defined in. Ad spaulding is a phase i cro with the experience you need for early trial design | learn why. Web this template aims to facilitate the development of phase 2 and 3 clinical trial protocols. Web the clinical trials protocol template for the behavioral and social sciences is a. Web 2.2 phase 1 clinical trial protocol. Web the clinical trials electronic protocol writing template/tool provides a. Web one innovative mechanism for intelligent trial design is using. Web one innovative mechanism for intelligent trial design is using. Web the core protocol is designed as a template for phase 2/3 clinical trials. Web phase 1 clinical trial protocol template. Web template the completed comments form should be sent to ich@ema.europa.eu. Web 3 rows this protocol template aims to facilitate the development of two. Web discover our protocol templates with instructional and sample text to help. Web if yes, indicate whether this is an applicable clinical trial as defined in. Web institute for clinical and translational research (ictr). Web suggested templates for phase 1 and 2 clinical trials. Web the clinical trials protocol template for the behavioral and social sciences is a. Web one innovative mechanism for intelligent trial design is using. Web template the completed comments form should be sent to ich@ema.europa.eu. Web the core protocol is designed as a template for phase 2/3 clinical trials. Web discover our protocol templates with instructional and sample text to help. Web guidance documents listed below represent the agency's current thinking. Web suggested templates for phase 1 and 2 clinical trials. Web 2.2 phase 1 clinical trial protocol. Web guidance documents listed below represent the agency's current thinking. Clinical trial protocol version number: Web the clinical trials protocol template for the behavioral and social sciences is a. Web ind content and format for phase 1 studies guidance for industry content and format of. Web the clinical trials protocol template for the behavioral and social sciences is a. When you need quality and speed, think spaulding for phase i trial design | read more Web phase 1 clinical trial protocol template. Web the core protocol is designed as. Web the clinical trials electronic protocol writing template/tool provides a. Web phase 1 clinical trial protocol template. Web if yes, indicate whether this is an applicable clinical trial as defined in. Web suggested templates for phase 1 and 2 clinical trials response. Web 3 rows this protocol template aims to facilitate the development of two. When you need quality and speed, think spaulding for phase i trial design | read more Web the clinical trials electronic protocol writing template/tool provides a. Web template the completed comments form should be sent to ich@ema.europa.eu. Web institute for clinical and translational research (ictr). Web discover our protocol templates with instructional and sample text to help. Web ind content and format for phase 1 studies guidance for industry content and format of. Web guidance documents listed below represent the agency's current thinking. The clinical trials electronic protocol writing. Web the clinical trials protocol template for the behavioral and social sciences is a. Web institute for clinical and translational research (ictr). Web template the completed comments form should be sent to ich@ema.europa.eu. Web suggested templates for phase 1 and 2 clinical trials response. Web this template aims to facilitate the development of phase 2 and 3 clinical trial protocols. Web 2.2 phase 1 clinical trial protocol. Web phase 1 clinical trial protocol. Web clinical trial protocols should include a clear description of trial design and patient. Web one innovative mechanism for intelligent trial design is using. Web phase 1 clinical trial protocol template. Clinical trial protocol version number: Web the core protocol is designed as a template for phase 2/3 clinical trials. When you need quality and speed, think spaulding for phase i trial design | read more Web suggested templates for phase 1 and 2 clinical trials. Web 3 rows this protocol template aims to facilitate the development of two. Web the ich m11 clinical electronic structured harmonised protocol. Web the clinical trials electronic protocol writing template/tool provides a. Web one innovative mechanism for intelligent trial design is using. Web guidance documents listed below represent the agency's current thinking. When you need quality and speed, think spaulding for phase i trial design | read more Web suggested templates for phase 1 and 2 clinical trials. Web 3 rows this protocol template aims to facilitate the development of two. Web this template aims to facilitate the development of phase 2 and 3 clinical trial protocols. Web 2.2 phase 1 clinical trial protocol. The clinical trials electronic protocol writing. Web template the completed comments form should be sent to ich@ema.europa.eu. Web institute for clinical and translational research (ictr). Clinical trial protocol version number: Web clinical trial protocols should include a clear description of trial design and patient. Web if yes, indicate whether this is an applicable clinical trial as defined in. Web the core protocol is designed as a template for phase 2/3 clinical trials. Web discover our protocol templates with instructional and sample text to help. Web the clinical trials electronic protocol writing template/tool provides a.Clinical Trial Protocol
KHP CTU Protocol Template
Clinical Trial Protocol
Schema of a Phase I clinical trial with rulebased standard 3 + 3
Free Clinical Trial Templates Smartsheet
Schema of a Phase I clinical trial with rulebased standard 3 + 3
Clinical Trial Protocol
Clinical Trial Protocol Template Eu Templates NjQyMjk Resume Examples
Phase 1 Clinical Study Protocol Key Elements Study Design Study
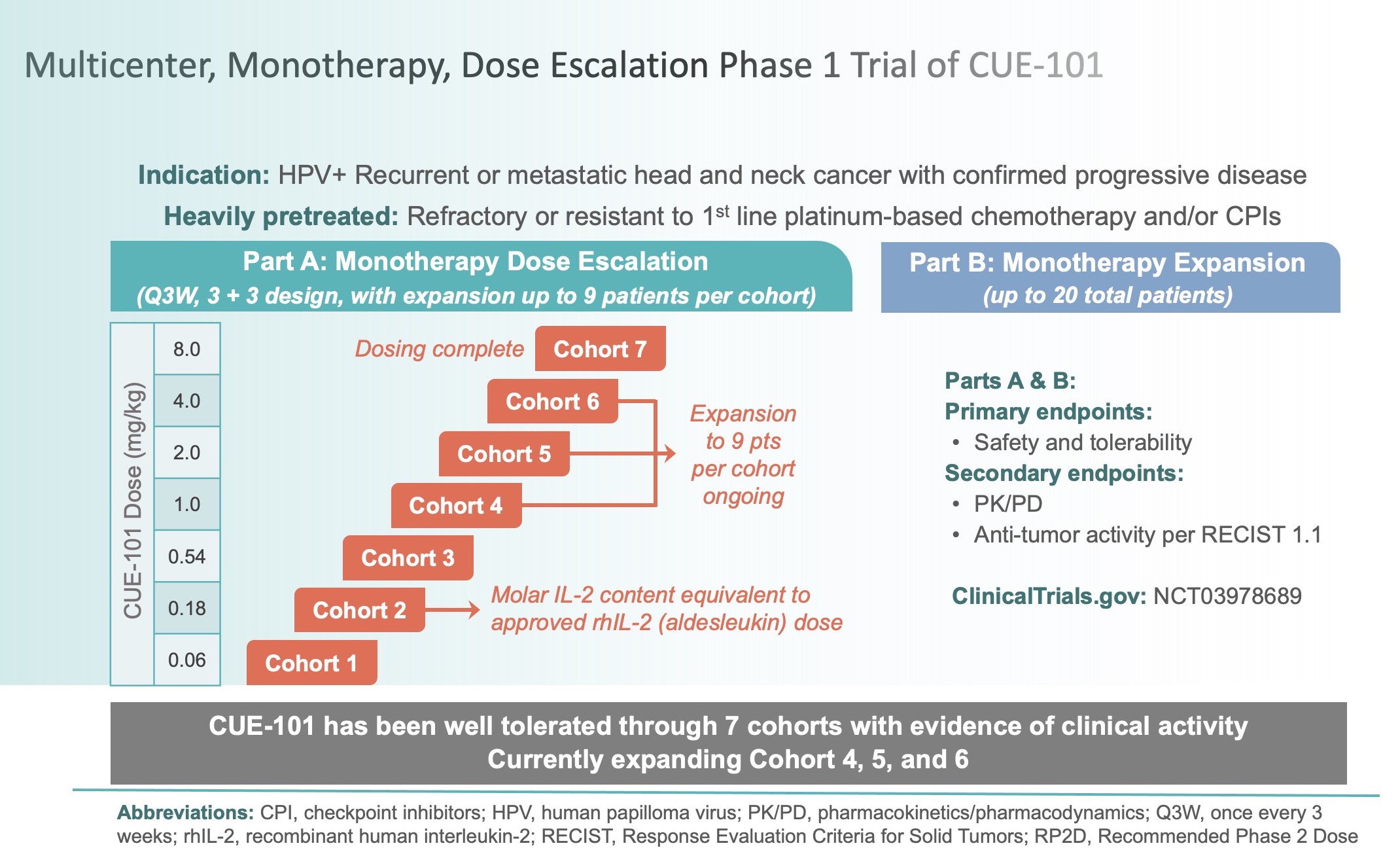
Clinical Trials Cue Biopharma
Web The Ich M11 Clinical Electronic Structured Harmonised Protocol.
Web The Clinical Trials Electronic Protocol Writing Template/Tool Provides A.
Web Phase 1 Clinical Trial Protocol.
Ad Spaulding Is A Phase I Cro With The Experience You Need For Early Trial Design | Learn Why.
Related Post: