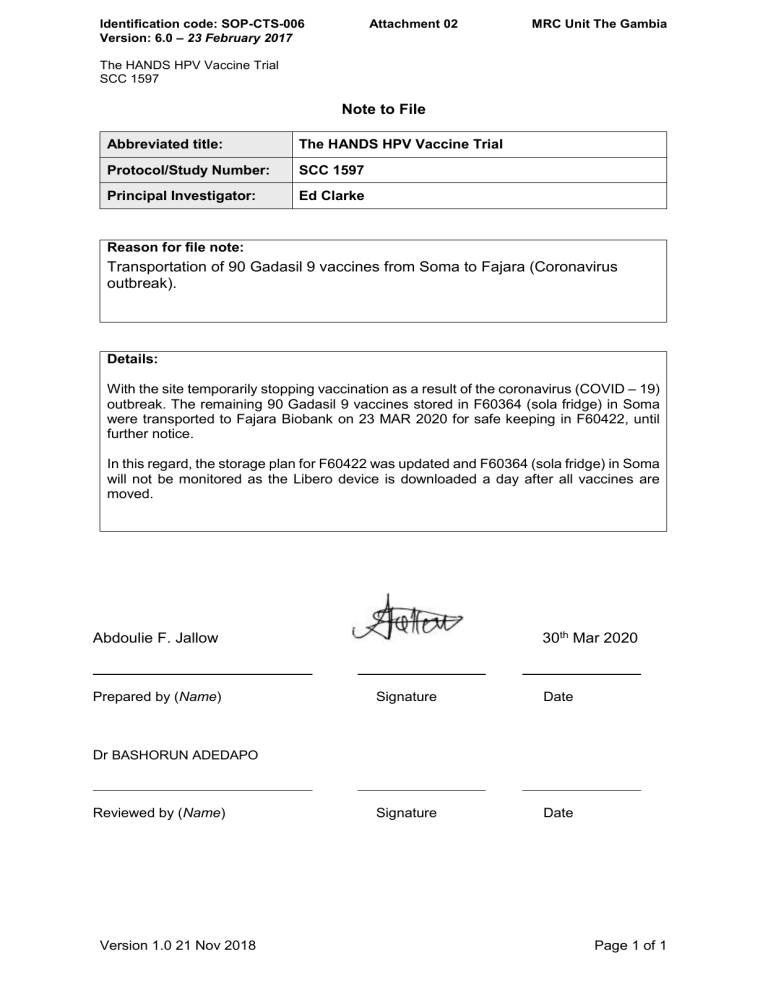
Note To File Template Clinical Research
Note To File Template Clinical Research - More than likely, you are writing and filing too many. Web the button contains templates, sample forms, guidelines, regulations and informational materials to supporting investigators at an company and conduct to high quality clinical. Web welcome to global health trials' tools and templates library. Example 1 (doc) example 2 (doc) example 3 (doc) Web the following page provides a template for the content and format of a note to the study file. Web valid notes to file (ntfs) should, at minimum, meet the following basic criteria: Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by nih that are being conducted under a fda ind or ide application. Kept on file in the site regulatory file and made available to the clinical site monitors reviewing the site’s. Include the subject and protocol to which it refers. Web november 30, 2015 this post is unconventional and perhaps a little unpopular, but it has to be said: Web healthcare business proposal templates. Web welcome to global health trials' tools and templates library. Web valid notes to file (ntfs) should, at minimum, meet the following basic criteria: Text enclosed with <> is a placeholder for a specific detail (e.g.,. Web note to file examples: Responsibility to be used by principal investigators and study team. I will provide, every 6 months, a written summary regarding data. Documents are important and essential in the healthcare and medical industries. The primary purpose of every tmf is to tell the story of a. More than likely, you are writing and filing too many. The primary purpose of every tmf is to tell the story of a. Responsibility to be used by principal investigators and study team. Web valid notes to file (ntfs) should, at minimum, meet the following basic criteria: This template assists the study team in contacting study participants. More than likely, you are writing and filing too many. Web the button contains templates, sample forms, guidelines, regulations and informational materials to supporting investigators at an company and conduct to high quality clinical. Web november 30, 2015 this post is unconventional and perhaps a little unpopular, but it has to be said: It is used to clarify an error, omission or discrepancy or to document a problem or corrective. Web this template provides a recommended structure for documenting note to files for research studies. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by nih that are being conducted under a fda ind or ide application. Please note that this page has been updated for 2015 following a quality check. Documents are important and essential in the healthcare and medical industries. Web nimh note to file (ntf) template version 1.0 nimh version 1.0 july 2019page 2of 2 nimh note t o file (ntf) template tool summary remove tool summary before. The primary purpose of every tmf is to tell the story of a. Web this template provides a recommended structure. The number of documents listed below includes. Text enclosed with <> is a placeholder for a specific detail (e.g.,. Kept on file in the site regulatory file and made available to the clinical site monitors reviewing the site’s. Web the button contains templates, sample forms, guidelines, regulations and informational materials to supporting investigators at an company and conduct to high. Web the following page provides a template for the content and format of a note to the study file. Web a note to the study file should be retained and stored, as follows: I will provide, every 6 months, a written summary regarding data. Responsibility to be used by principal investigators and study team. Web note to file examples: Web november 30, 2015 this post is unconventional and perhaps a little unpopular, but it has to be said: Web healthcare business proposal templates. Regulatory binder pointer page templates; Web regulatory binder protocol deviation log template; Web the following page provides a template for the content and format of a note to the study file. Web valid notes to file (ntfs) should, at minimum, meet the following basic criteria: Regulatory binder note to file templates; The number of documents listed below includes. Web november 30, 2015 this post is unconventional and perhaps a little unpopular, but it has to be said: Text enclosed with <> is a placeholder for a specific detail (e.g.,. Documents are important and essential in the healthcare and medical industries. The number of documents listed below includes. Kept on file in the site regulatory file and made available to the clinical site monitors reviewing the site’s. Please note that this page has been updated for 2015 following a quality check and review of the templates, and many. Web valid notes to file (ntfs) should, at minimum, meet the following basic criteria: Web research findings and/or have a delegate of the bcca vp of research sit in on research study meetings. I will provide, every 6 months, a written summary regarding data. The primary purpose of every tmf is to tell the story of a. Web welcome to global health trials' tools and templates library. This template assists the study team in contacting study participants. Web clinical study tools and templates. Web this template provides a recommended structure for documenting note to files for research studies. Text enclosed with <> is a placeholder for a specific detail (e.g.,. Regulatory binder pointer page templates; Web nimh note to file (ntf) template version 1.0 nimh version 1.0 july 2019page 2of 2 nimh note t o file (ntf) template tool summary remove tool summary before. Web healthcare business proposal templates. Example 1 (doc) example 2 (doc) example 3 (doc) Web the button contains templates, sample forms, guidelines, regulations and informational materials to supporting investigators at an company and conduct to high quality clinical. More than likely, you are writing and filing too many. Web november 30, 2015 this post is unconventional and perhaps a little unpopular, but it has to be said: This template assists the study team in contacting study participants. Web clinical study tools and templates. Responsibility to be used by principal investigators and study team. Web this template provides a recommended structure for documenting note to files for research studies. Text enclosed with <> is a placeholder for a specific detail (e.g.,. Web november 30, 2015 this post is unconventional and perhaps a little unpopular, but it has to be said: Web nimh note to file (ntf) template version 1.0 nimh version 1.0 july 2019page 2of 2 nimh note t o file (ntf) template tool summary remove tool summary before. More than likely, you are writing and filing too many. Web note to file examples: Please note that this page has been updated for 2015 following a quality check and review of the templates, and many. Web a note to the study file should be retained and stored, as follows: I will provide, every 6 months, a written summary regarding data. Web welcome to global health trials' tools and templates library. Web the button contains templates, sample forms, guidelines, regulations and informational materials to supporting investigators at an company and conduct to high quality clinical. Regulatory binder note to file templates; Web healthcare business proposal templates.Clinical Trial Report Template (1) TEMPLATES EXAMPLE TEMPLATES
2019 Pediatric Clinical Note Template2 Medical Diagnosis Health Care
PPT Orientation for New Clinical Research PERSONNEL Module 2
Note To File Template Download by Pharma Student Issuu
Clinical Progress Note Template
181121 File Note template
Note To File Sample HQ Printable Documents
FREE 38+ Notes Samples in Google Docs MS Word Apple Pages
Clinical Progress Note Format
Editable Clinical Progress Note Template Counseling Dap inside Dap Note
Web Research Findings And/Or Have A Delegate Of The Bcca Vp Of Research Sit In On Research Study Meetings.
Web Valid Notes To File (Ntfs) Should, At Minimum, Meet The Following Basic Criteria:
Include The Subject And Protocol To Which It Refers.
Kept On File In The Site Regulatory File And Made Available To The Clinical Site Monitors Reviewing The Site’s.
Related Post: