Medical Device Verification And Validation Plan Template
Medical Device Verification And Validation Plan Template - Web their complete medical device qms template package is available for $875, and it contains 28 quality procedures, 16 quality forms, the quality manual, and 24 other templates and logs. We lack experience in this area and to see an example of how this should be done would be incredibly helpful. Web studying precis what design verification and design validated are, how they are the same, how they are different, and best practices for medical devices. Download the entire series in one convenient pdf. Did we make what we said we would make? Web verification and validation aspects of specified design envelope and medical device production system authoring group: Web a master validation plan (mvp) is simply a plan for your equipment and process validation activities. Define equipment and processes to which these guidelines apply, step 2: Prepare and document the validation plan and test runs by specific process and / or equipment, step 4: Trusted by leading pharma, biotech, and medical device companies globally. Did we make what we said we would make? 10 august 2022 tracey duffy, imdrf chair this document was produced by the international medical device regulators forum. Web jun 20, 2019 #1 dear all, does anybody have a template or example for verification and validation activities and associated testing plan for 13485 (no software)? Web this guidance outlines general validation. Web safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements for medical devices. The plan should reference the applicable protocol and report for each item in the plan. Any set of criteria can be subjected to verification. 10 august 2022 tracey duffy, imdrf chair this document was produced by. Define equipment and processes to which these guidelines apply, step 2: 21 cfr 820.30 design controls (f) design verification. The purpose of the record is to develop a plan forward validation and authentication related. It requires you to document each of these design outputs because they are evidence you met the design inputs. Package consists of the procedure and a. Any set of criteria can be subjected to verification. Web quality system regulation process validation fda small business regulatory education for industry (redi) silver spring md september 30, 2015 joseph tartal Web their complete medical device qms template package is available for $875, and it contains 28 quality procedures, 16 quality forms, the quality manual, and 24 other templates and. Web a master validation plan (mvp) is simply a plan for your equipment and process validation activities. Web safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements for medical devices. Define equipment and processes to which these guidelines apply, step 2: Web this guidance outlines general validation principles that. As an added bonus, med dev qms will refund the entire purchase price if you’re not 100%. Web verification is the process of ensuring your medical device satisfies the design inputs. Did we make what we said we would make? Any set of criteria can be subjected to verification. Web a master validation plan (mvp) is simply a plan for. The plan should reference the applicable protocol and report for each item in the plan. The device is a basic stainless steel instrument. All the equipment, processes, and software requiring validation should be included in the mvp. Download the entire series in one convenient pdf. It requires you to document each of these design outputs because they are evidence you. Any set of criteria can be subjected to verification. Define equipment and processes to which these guidelines apply, step 2: Did we make what we said we would make? The purpose of the record is to develop a plan forward validation and authentication related. Validation 3.8.13 (bs en iso 9001:2015) Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. Validation 3.8.13 (bs en iso 9001:2015) Web verification and validation aspects of specified design envelope and medical device production system authoring group: Ad digitize and manage any validation, commissioning or qualification process. Web a master validation. The plan should reference the applicable protocol and report for each item in the plan. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. 21 cfr 820.30 design controls (f) design verification. Define validation objectives and hypotheses, step 3: At some point in the new. ⇓ download this article as pdf. Download the entire series in one convenient pdf. 10 august 2022 tracey duffy, imdrf chair this document was produced by the international medical device regulators forum. Web the purpose of the start is until develop a plan available endorsement and verification activities in the design and technology process. Web safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements for medical devices. Web verification is the process of ensuring your medical device satisfies the design inputs. 21 cfr 820.30 design controls (f) design verification. As an added bonus, med dev qms will refund the entire purchase price if you’re not 100%. Execute necessary test runs and record results, Package consists of the procedure and a design review report form. We lack experience in this area and to see an example of how this should be done would be incredibly helpful. Web medical device design verification essentials. Web their complete medical device qms template package is available for $875, and it contains 28 quality procedures, 16 quality forms, the quality manual, and 24 other templates and logs. As with other options, the files come in either word or excel format. The plan should reference the applicable protocol and report for each item in the plan. All the equipment, processes, and software requiring validation should be included in the mvp. The purpose of the record is to develop a plan forward validation and authentication related. Ad digitize and manage any validation, commissioning or qualification process. Web verification and validation aspects of specified design envelope and medical device production system authoring group: It requires you to document each of these design outputs because they are evidence you met the design inputs. In our first post we covered the basics of process validation, and in subsequent posts we cover iq, oq, pq, and revalidation. Web verification is the process of ensuring your medical device satisfies the design inputs. Web this guidance outlines general validation principles that the food and drug administration (fda) considers to be applicable to the validation of medical device software or the validation. As an added bonus, med dev qms will refund the entire purchase price if you’re not 100%. The purpose of the record is to develop a plan forward validation and authentication related. Validation 3.8.13 (bs en iso 9001:2015) We lack experience in this area and to see an example of how this should be done would be incredibly helpful. 10 august 2022 tracey duffy, imdrf chair this document was produced by the international medical device regulators forum. Define validation objectives and hypotheses, step 3: Web a master validation plan (mvp) is simply a plan for your equipment and process validation activities. As with other options, the files come in either word or excel format. The plan should reference the applicable protocol and report for each item in the plan. The device is a basic stainless steel instrument. ⇓ download this article as pdf. Web studying precis what design verification and design validated are, how they are the same, how they are different, and best practices for medical devices. Prepare and document the validation plan and test runs by specific process and / or equipment, step 4:Verification and Validation Plan Template (MS Word) Templates, Forms
10+ Validation Plan Templates Sample Templates
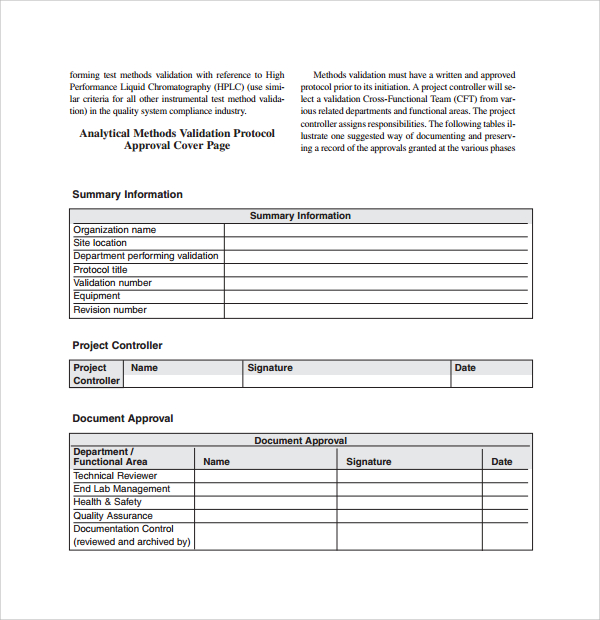
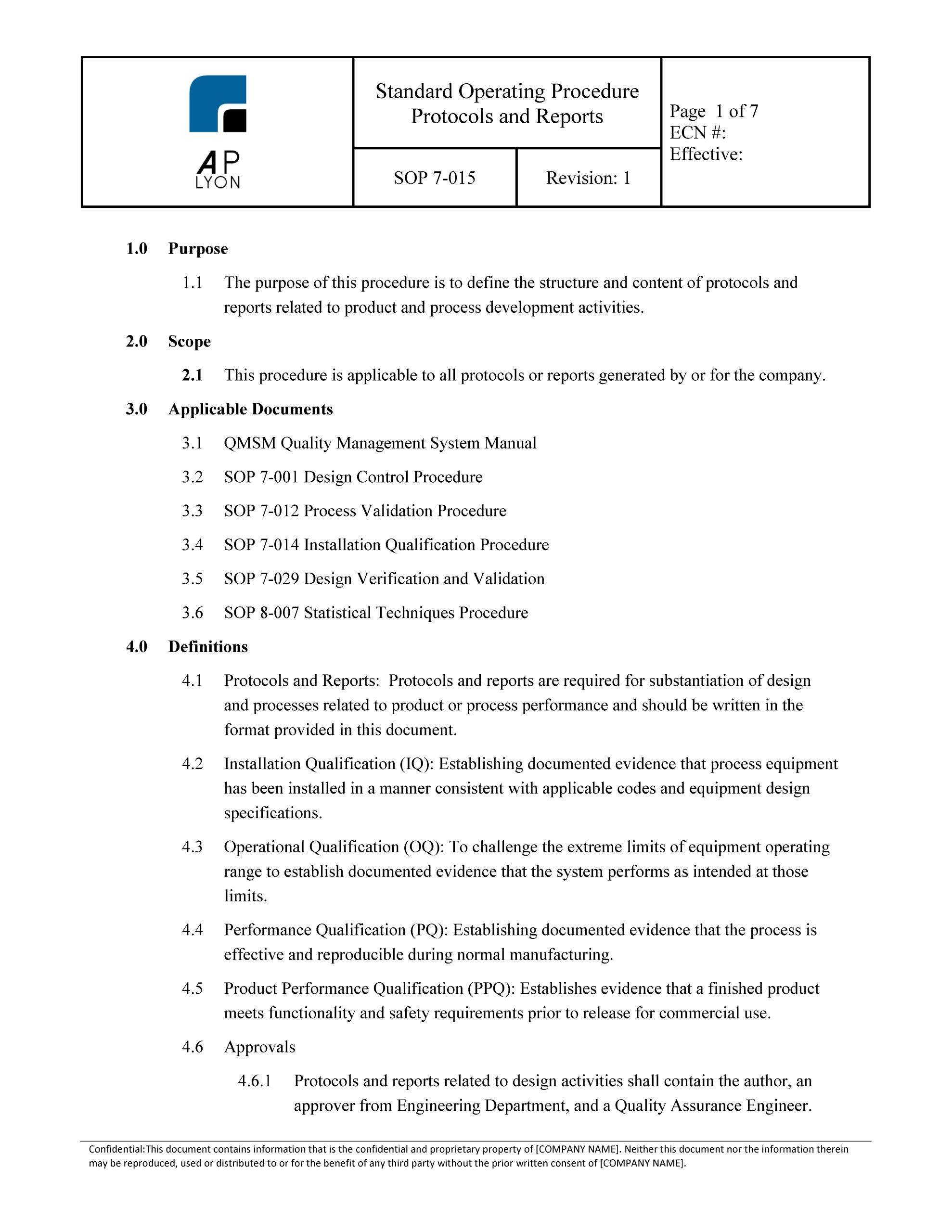
Validation Protocols Reports Procedure
Verification and Validation Plan Template (MS Word) Templates, Forms
Template of a validation plan. Download Scientific Diagram
Medical Device Design Verification SOP
Template Word Master Software Validation Test Plan according to the
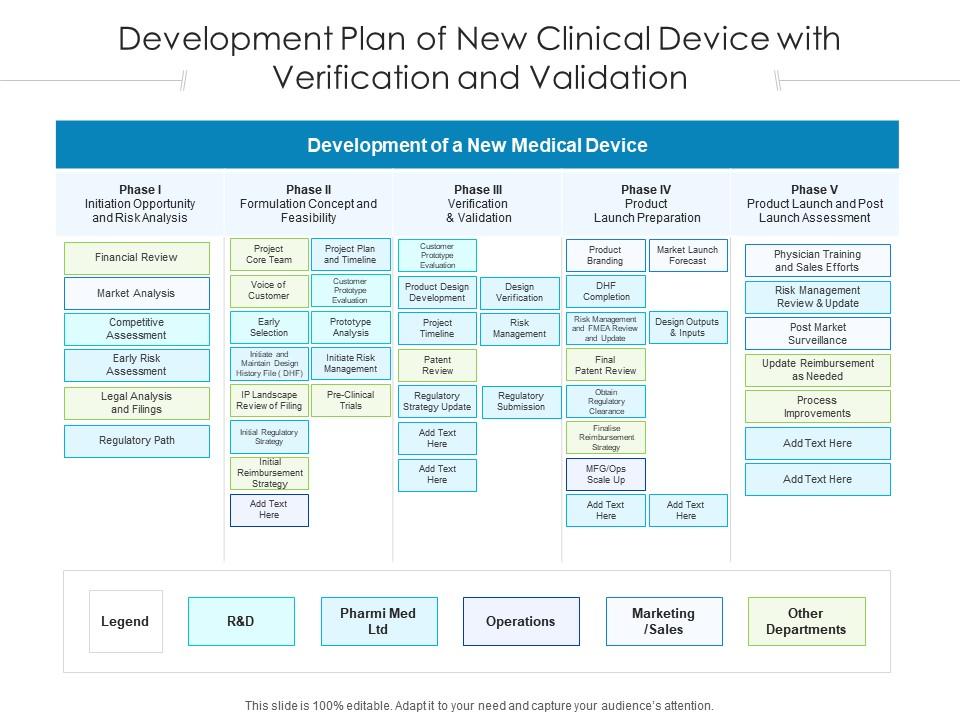
Development Plan Of New Clinical Device With Verification And
PROCESS VALIDATION SOP Template MD46 GMP, QSR & ISO Compliance
Conducting Medical Device Verification and Validation Tests
Download The Entire Series In One Convenient Pdf.
Any Set Of Criteria Can Be Subjected To Verification.
Define Equipment And Processes To Which These Guidelines Apply, Step 2:
Trusted By Leading Pharma, Biotech, And Medical Device Companies Globally.
Related Post: