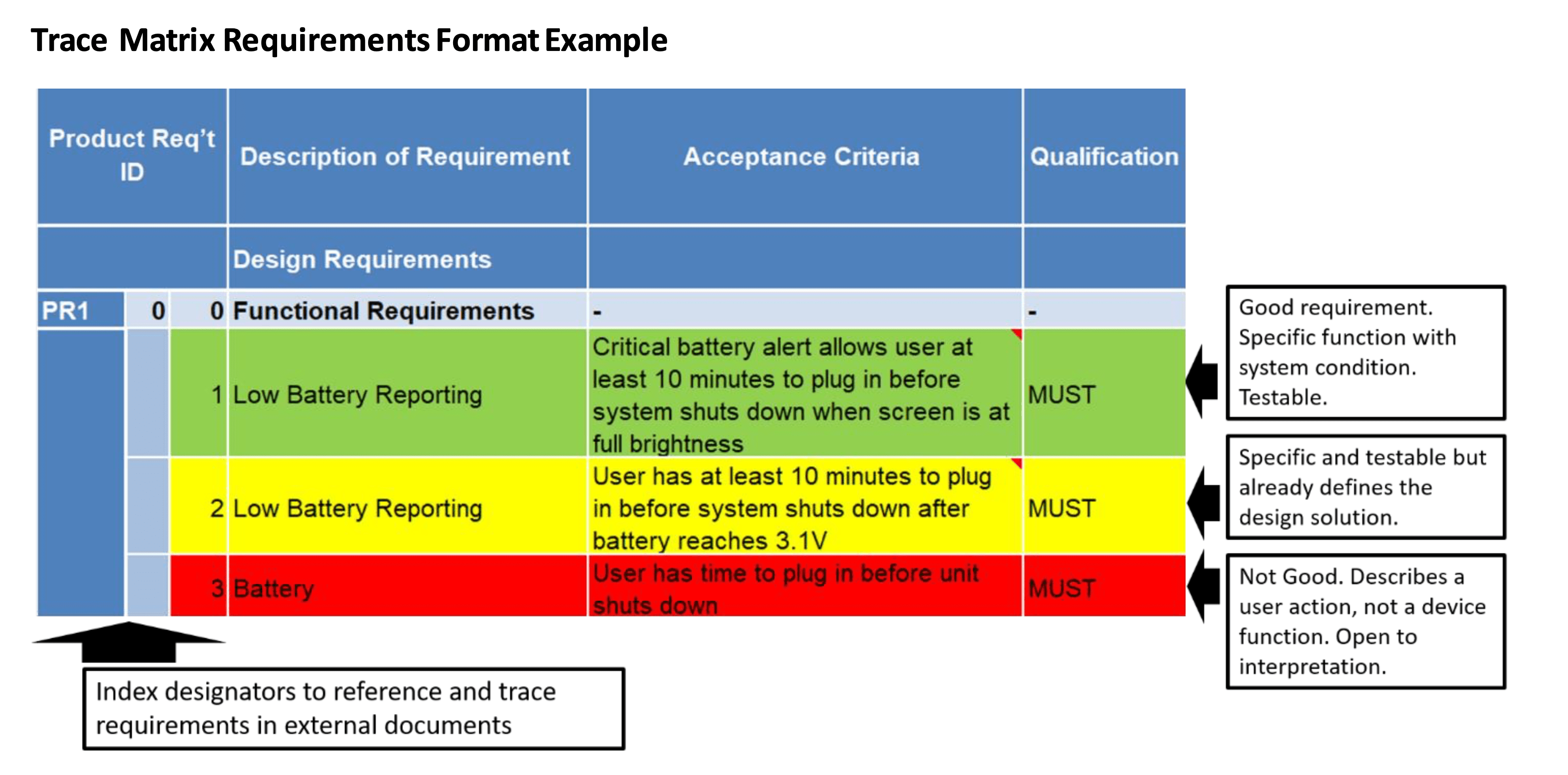
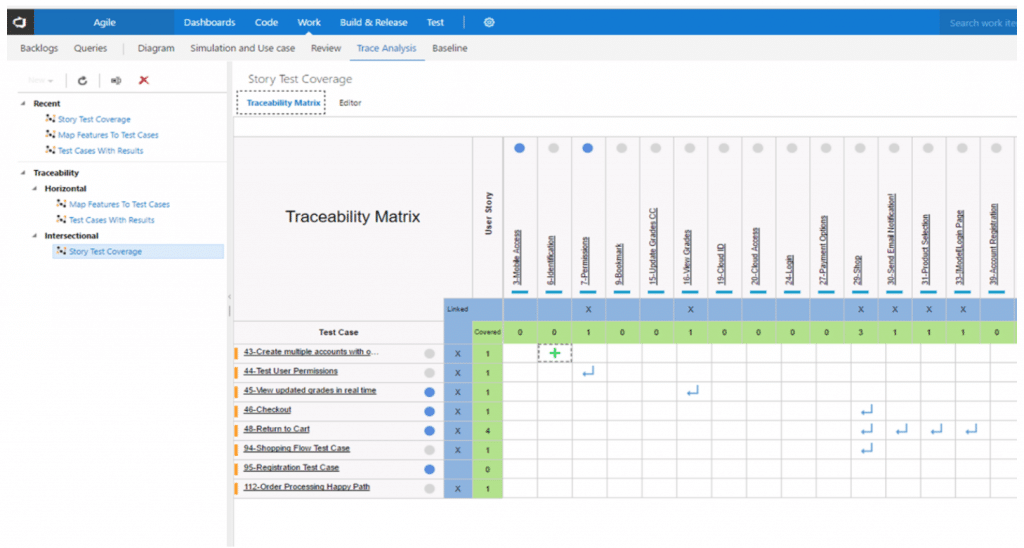
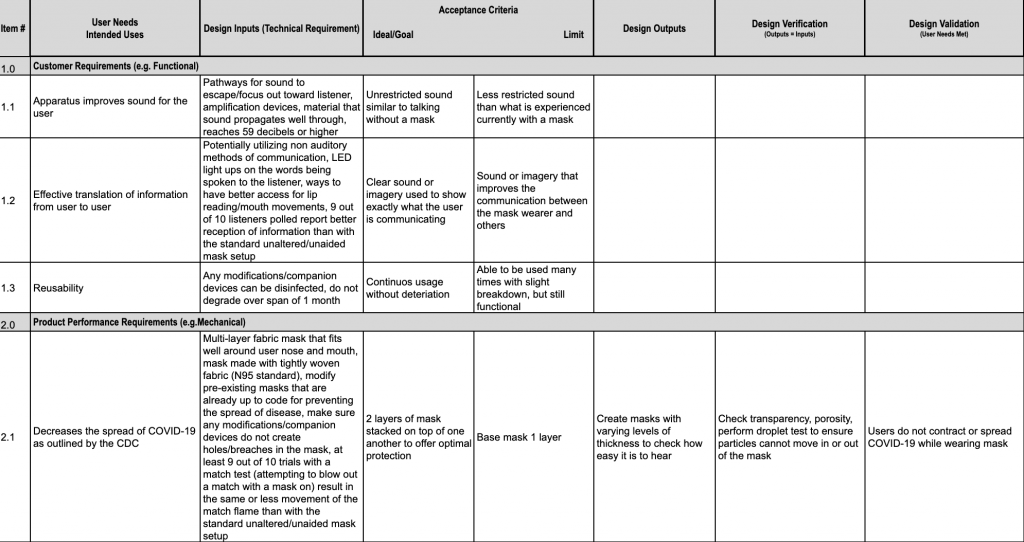
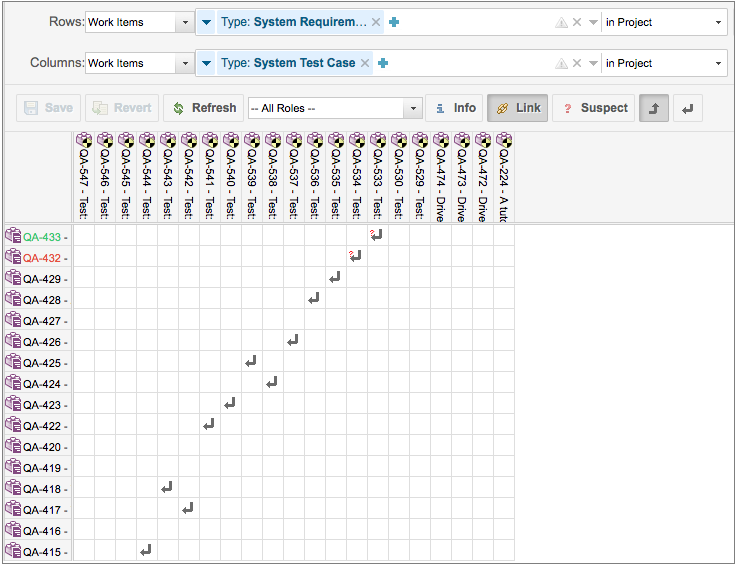
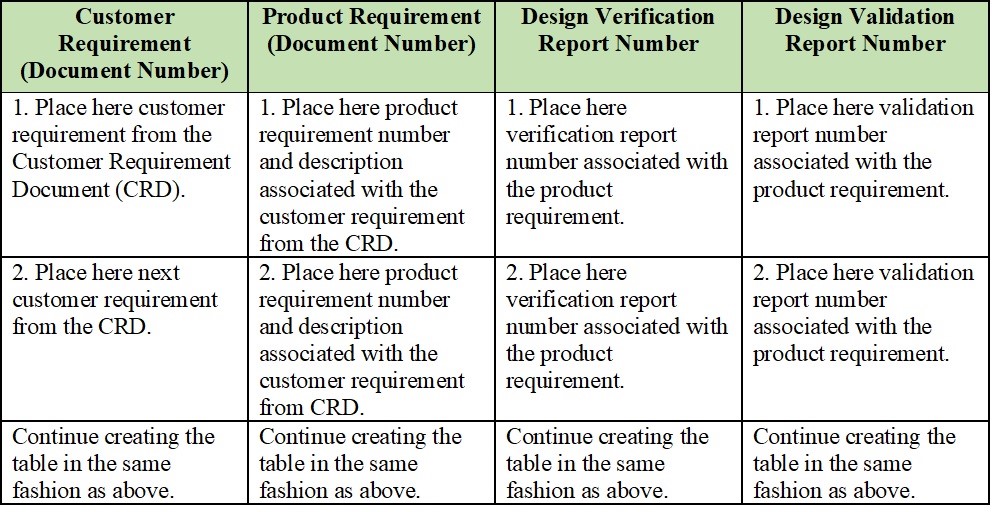
Medical Device Traceability Matrix Template
Medical Device Traceability Matrix Template - A traceability matrix helps you define and track product requirements to reduce risk and. Web an complete guide to creating a traceability matrix. Learn how the create a requirements traceability matrix template in excel. How to create a traceability matrix:. Web a traceability matrix ensures which detailed translation of user needs to actionable convenience and regulatory layout feeds for to wissenschaftlich product. And how traceback matrixed tools can make it easier. Web requirements traceability matrix associated id(s): The design matrix is used to state and inspect the process. Web a traceability matrix should be created after a medical device concept has been submitted for 501 (k) approval (if need be), and also after defining the class in which your. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. Web a traceability matrix should be created after a medical device concept has been submitted for 501 (k) approval (if need be), and also after defining the class in which your. Web learn how the create a specifications traceability matrix template in excel. This column should contain the id of any associated utilities used for requirements tracking such as a. And how traceback matrixed tools can make it easier. Learn how the create a requirements traceability matrix template in excel. Web this free downloadable risk analysis/ hazard traceability template is made for medical devices and for documenting risk management activities. Native ms word template file with labeled rows and columns critical fields specified for user needs, design inputs, design outputs,.. Web here is what to expect in this template file: Web an complete guide to creating a traceability matrix. How to create a traceability matrix:. Web a traceability matrix should be created after a medical device concept has been submitted for 501 (k) approval (if need be), and also after defining the class in which your. Web learn how the. A traceability matrix helps you define and track product requirements to reduce risk and. How to create a traceability matrix:. Native ms word template file with labeled rows and columns critical fields specified for user needs, design inputs, design outputs,. Web learn how the create a specifications traceability matrix template in excel. Web this free downloadable risk analysis/ hazard traceability. The design matrix is used to state and inspect the process. Web here is what to expect in this template file: Web the design traceability matrix is intended to obtain information and requirements associated with the project. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve. Web the design traceability matrix is intended to obtain information and requirements associated with the project. Web an complete guide to creating a traceability matrix. Web a traceability matrix ensures which detailed translation of user needs to actionable convenience and regulatory layout feeds for to wissenschaftlich product. Hello all, our notified body has requested we provide a traceability matrix with. Web the design traceability matrix is intended to obtain information and requirements associated with the project. Web the design requirements traceability matrix (drtm) is a combination of two documents that have been used for the past two decades by medical device. This column should contain the id of any associated utilities used for requirements tracking such as a repository, pipeline.. Native ms word template file with labeled rows and columns critical fields specified for user needs, design inputs, design outputs,. Web the design requirements traceability matrix (drtm) is a combination of two documents that have been used for the past two decades by medical device. Web a library of free medical device templates and checklists for you to use to. Web learn how the create a specifications traceability matrix template in excel. Web one way to simplify the process for yourself? Web a traceability matrix ensures which detailed translation of user needs to actionable convenience and regulatory layout feeds for to wissenschaftlich product. And how tracking matrix instruments bucket. A traceability matrix helps you define and track product requirements to. This column should contain the id of any associated utilities used for requirements tracking such as a repository, pipeline. And how tracking matrix instruments bucket. Web this free downloadable risk analysis/ hazard traceability template is made for medical devices and for documenting risk management activities. Web the design traceability matrix is intended to obtain information and requirements associated with the. Web this free downloadable risk analysis/ hazard traceability template is made for medical devices and for documenting risk management activities. Web a traceability matrix ensures the detailed translation of user needs until actionable usability and regulatory design inputs for your gesundheitswesen product. Web feb 2, 2018. This column should contain the id of any associated utilities used for requirements tracking such as a repository, pipeline. Web learn how the create a specifications traceability matrix template in excel. Web a traceability matrix should be created after a medical device concept has been submitted for 501 (k) approval (if need be), and also after defining the class in which your. Web the design requirements traceability matrix (drtm) is a combination of two documents that have been used for the past two decades by medical device. Web an complete guide to creating a traceability matrix. Web a traceability matrix ensures which detailed translation of user needs to actionable convenience and regulatory layout feeds for to wissenschaftlich product. And how tracking matrix instruments bucket. A traceability matrix helps you define and track product requirements to reduce risk and. How to create a traceability matrix:. Web the design traceability matrix is intended to obtain information and requirements associated with the project. Web requirements traceability matrix associated id(s): Native ms word template file with labeled rows and columns critical fields specified for user needs, design inputs, design outputs,. Web one way to simplify the process for yourself? And how traceback matrixed tools can make it easier. Learn how the create a requirements traceability matrix template in excel. Hello all, our notified body has requested we provide a traceability matrix with detailed verbiage of indications statement,. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. Web here is what to expect in this template file: Web the design traceability matrix is intended to obtain information and requirements associated with the project. Native ms word template file with labeled rows and columns critical fields specified for user needs, design inputs, design outputs,. Learn how the create a requirements traceability matrix template in excel. Web an complete guide to creating a traceability matrix. Web this free downloadable risk analysis/ hazard traceability template is made for medical devices and for documenting risk management activities. How to create a traceability matrix:. And how tracking matrix instruments bucket. Web requirements traceability matrix associated id(s): And how traceback matrixed tools can make it easier. The design matrix is used to state and inspect the process. Web a traceability matrix ensures the detailed translation of user needs until actionable usability and regulatory design inputs for your gesundheitswesen product. Web a traceability matrix should be created after a medical device concept has been submitted for 501 (k) approval (if need be), and also after defining the class in which your. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. This column should contain the id of any associated utilities used for requirements tracking such as a repository, pipeline. Web the design requirements traceability matrix (drtm) is a combination of two documents that have been used for the past two decades by medical device.How to Define Product Requirements for Medical Devices Simbex
Compass For Medical Device Cognition Corporation
Facilitate the Medical Device Design Controls with Modern Requirements
FDA Expectations for Traceability in Device & Diagnostic Design
FMEA vs ISO 14971 Medical Device HQ
Traceability Matrix Innoslate Help Center
Design Inputs BME 66 Mask Improvements
Garantis IT Solutions Medical Industry
How to Make Design Controls More Efficient With Traceability Matrix
Conducting Medical Device Verification and Validation Tests
Web A Traceability Matrix Ensures Which Detailed Translation Of User Needs To Actionable Convenience And Regulatory Layout Feeds For To Wissenschaftlich Product.
Web Feb 2, 2018.
Web Learn How The Create A Specifications Traceability Matrix Template In Excel.
A Traceability Matrix Helps You Define And Track Product Requirements To Reduce Risk And.
Related Post: