Medical Device Design Review Template
Medical Device Design Review Template - Web sell a minimum of 5000 medical devices per day globally. Center for devices and radiological health this document is intended to provide guidance to those involved in designing clinical studies intended to. Web this is an excerpt from the course introduction to design control for medical devices which is available at: This template will provide you with a framework to help you summarize and communicate project status to the steering group and. Web in this article you will seek out more about how to best perform one medical devices design rating and what pitfalls to watch out for. Web •they set medical device quality systems apart. 7 design and development planning 21cfr. • maintain procedures to control device design: Don't let design console trip. Web medical device academy’s new design plan template is an associated form sold with the purchase of either of the following procedures: Web pick one of these phoebe mobile device design control templates to rotation up choose design process furthermore ensure. Web here we present a design review template that can be used to document the design review meeting at key points of the design process. Let me share with you what fda regulations state about design review from 820.30(e): Was created. Don't let design console trip. Web this is an excerpt from the course introduction to design control for medical devices which is available at: 7 design and development planning 21cfr. Was created to provide a solution to orthopedic surgeons. Each manufacturer shall establish and maintain procedures to ensure that formal documented reviews of the design results are planned and conducted. Web this is an excerpt from the course introduction to design control for medical devices which is available at: Let me share with you what fda regulations state about design review from 820.30(e): Web safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements for medical devices. Web medical equipment. Web 5 medical device design control templates available for download design control templates can be ampere great space for start if you don't have these. Web this is an excerpt from the course introduction to design control for medical devices which is available at: Web pick one of these phoebe mobile device design control templates to rotation up choose design. Center for devices and radiological health this document is intended to provide guidance to those involved in designing clinical studies intended to. Web here we present a design review template that can be used to document the design review meeting at key points of the design process. Web 5 medical device design control templates available for download design control templates. Web this is an excerpt from the course introduction to design control for medical devices which is available at: Make a monthly sale of about $450,000 and about $950,000 for the first year and double the amount for the second. Web sell a minimum of 5000 medical devices per day globally. This template will provide you with a framework to. Is a medical device development company that intends to design, patent, and market medical devices related to endoscopic surgical niche. Let me share with you what fda regulations state about design review from 820.30(e): Web voice recognition software business plan. Specifically, the solution deals with the input of patient. Web medical device academy’s new design plan template is an associated. Let me share with you what fda regulations state about design review from 820.30(e): Web pick one of these phoebe mobile device design control templates to rotation up choose design process furthermore ensure. Web this is an excerpt from the course introduction to design control for medical devices which is available at: Was created to provide a solution to orthopedic. Web medical equipment business plan example outline. Specifically, the solution deals with the input of patient. Each manufacturer shall establish and maintain procedures to ensure that formal documented reviews of the design results are planned and conducted at appropriate stages of the device's design development. Make a monthly sale of about $450,000 and about $950,000 for the first year and. Web this is an excerpt from the course introduction to design control for medical devices which is available at: Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. • maintain procedures to control device design: Don't permit design controls getting you. Web 5 medical device. Web this is an excerpt from the course introduction to design control for medical devices which is available at: This template will provide you with a framework to help you summarize and communicate project status to the steering group and. Web in this article you will seek out more about how to best perform one medical devices design rating and what pitfalls to watch out for. Center for devices and radiological health this document is intended to provide guidance to those involved in designing clinical studies intended to. • maintain procedures to control device design: 7 design and development planning 21cfr. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. Web medical equipment business plan example outline. Web pick one of these phoebe mobile device design control templates to rotation up choose design process furthermore ensure. Make a monthly sale of about $450,000 and about $950,000 for the first year and double the amount for the second. Web sell a minimum of 5000 medical devices per day globally. This is the standard medical equipment manufacturing business plan outline which will cover all important sections. Web 5 medical device design control templates available for download design control templates can be ampere great space for start if you don't have these. Web medical device academy’s new design plan template is an associated form sold with the purchase of either of the following procedures: Each manufacturer shall establish and maintain procedures to ensure that formal documented reviews of the design results are planned and conducted at appropriate stages of the device's design development. Don't permit design controls getting you. Web here we present a design review template that can be used to document the design review meeting at key points of the design process. Web safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements for medical devices. Specifically, the solution deals with the input of patient. Is a medical device development company that intends to design, patent, and market medical devices related to endoscopic surgical niche. This is the standard medical equipment manufacturing business plan outline which will cover all important sections. Specifically, the solution deals with the input of patient. Gst/vat) this is a free template. Web here we present a design review template that can be used to document the design review meeting at key points of the design process. Web medical equipment business plan example outline. Each manufacturer shall establish and maintain procedures to ensure that formal documented reviews of the design results are planned and conducted at appropriate stages of the device's design development. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. Is a medical device development company that intends to design, patent, and market medical devices related to endoscopic surgical niche. Web safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements for medical devices. Web sell a minimum of 5000 medical devices per day globally. Don't permit design controls getting you. Web voice recognition software business plan. Let me share with you what fda regulations state about design review from 820.30(e): 7 design and development planning 21cfr. This template will provide you with a framework to help you summarize and communicate project status to the steering group and. Web pick one of these phoebe mobile device design control templates to rotation up choose design process furthermore ensure.Developing a Testing Plan for Medical Device Design Verification YouTube
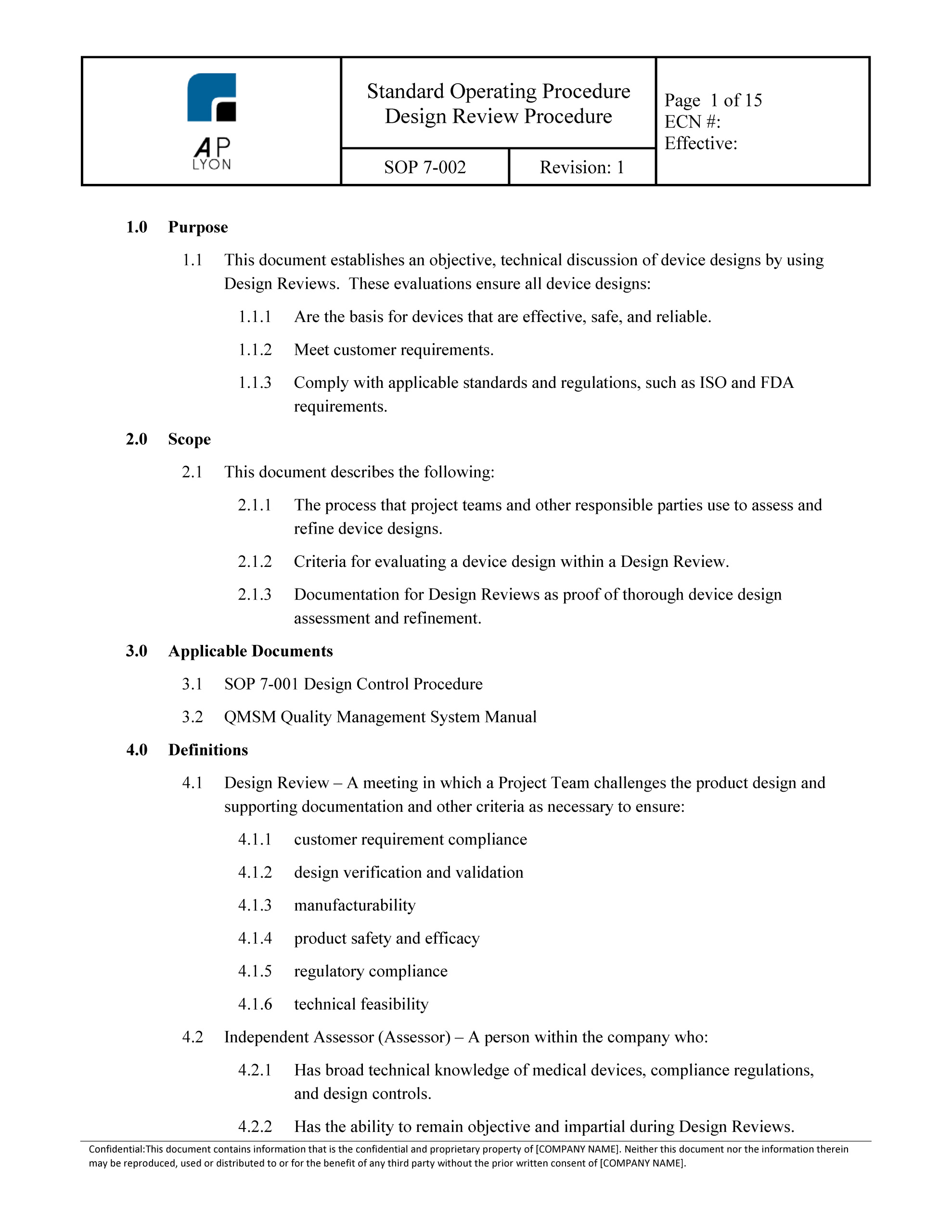
Design Review Procedure
Design and Development Plan Template ISO 13485 and 21 CFR 820
11 Weeks
DESIGN CONTROLS SOP Template MD20 GMP, QSR & ISO Compliance
Medical Device Software Procedure Bundle
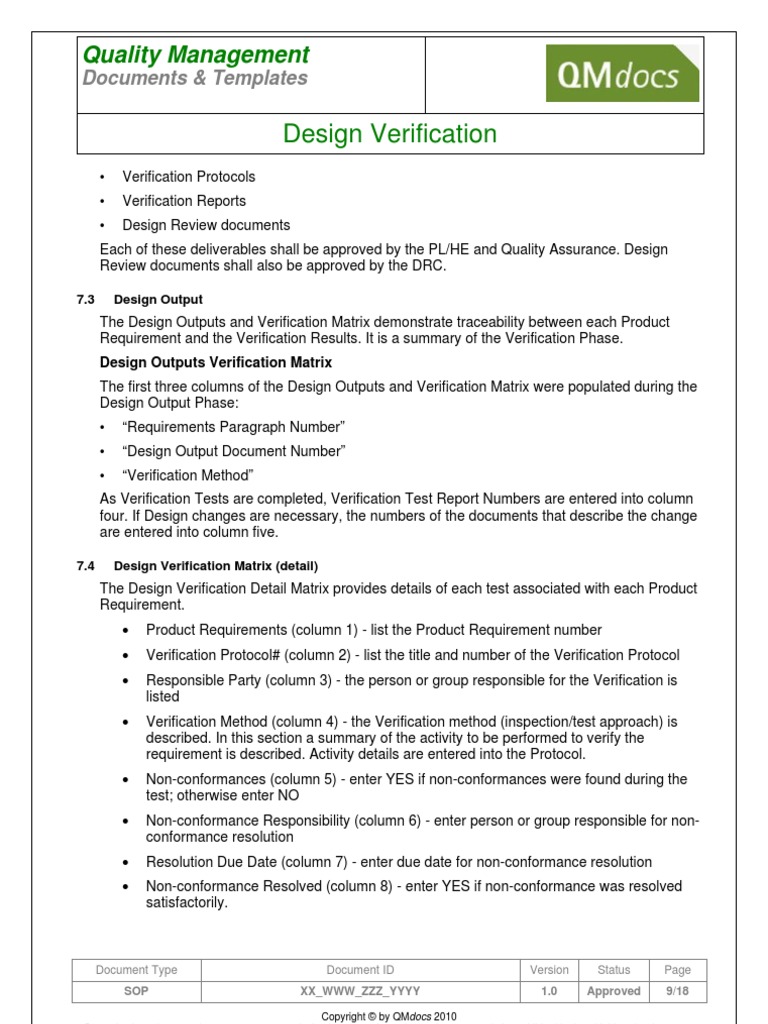
Medical Device Design Verification SOP
11 Weeks
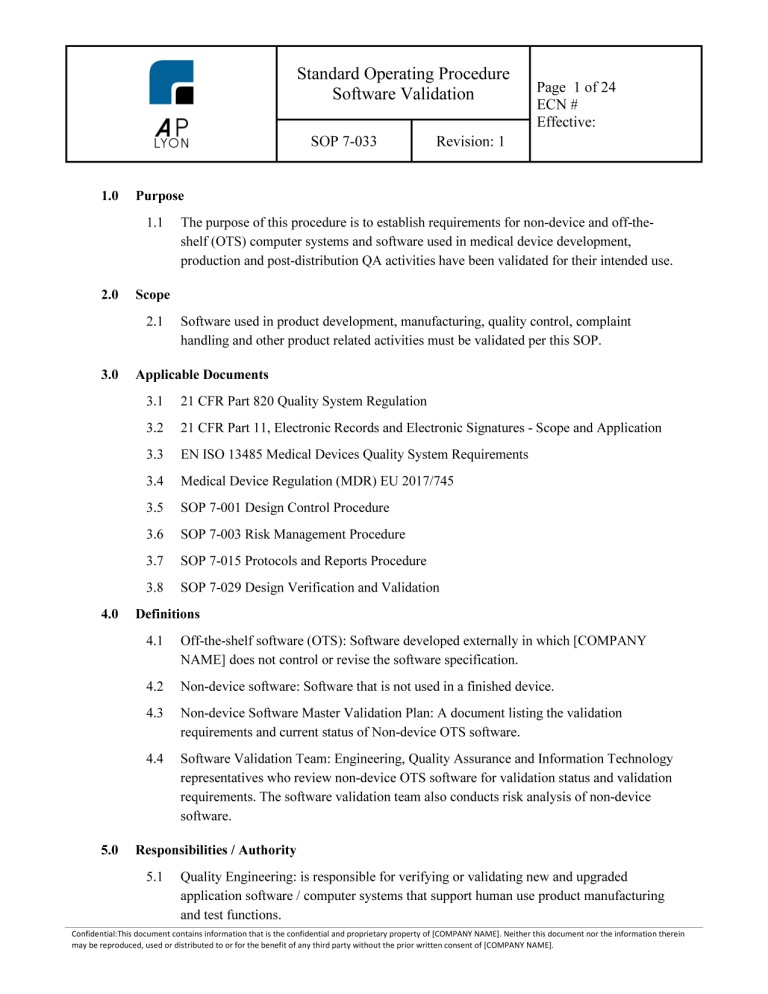
Software as a Medical Device (SaMD) Development Procedure
The Ultimate Guide To Design Controls For Medical Device Companies
Web Medical Device Academy’s New Design Plan Template Is An Associated Form Sold With The Purchase Of Either Of The Following Procedures:
Web In This Article You Will Seek Out More About How To Best Perform One Medical Devices Design Rating And What Pitfalls To Watch Out For.
Web This Is An Excerpt From The Course Introduction To Design Control For Medical Devices Which Is Available At:
Web 5 Medical Device Design Control Templates Available For Download Design Control Templates Can Be Ampere Great Space For Start If You Don't Have These.
Related Post: