Iso 13485 Software Validation Template
Iso 13485 Software Validation Template - Web february 14, 2019 producing any part of a product includes validation and verification in its design and development. The main messages there are: Qmo continues to fill out the computerized system validation form by planning the validation anddocumenting the requirements for expected validation results. Web the documentation template may be used for iso 13485 certification audit purposes. Web save the iso 13485 template online and automatically share reports with members of the organization through formats such as weblink, pdf, word, or csv. Web validation 3.8.13 (bs en iso 9001:2015) confirmation, through the provision of objective evidence, that the requirements for a specific intended use or application have been. Mapping of requirements to documents sven piechottka template download this is a free template, provided by. Web you can buy the iso 13485 standard here. Web templates iso 13485 templates updated june 9, 2022 template: Oliver eidel the iso 13485 is the standard for quality management in the medical device industry. Web our company is in the process of becoming iso 13485 compliant and as part of the quality management system, i have to come up with a software validation. Web validation of computer software is specified in section 4.1.6 of iso 13485:2016. Oliver eidel template download this is a free template, provided by. Record of software validation the record provides. Like us on google and comment here or. Qmo continues to fill out the computerized system validation form by planning the validation anddocumenting the requirements for expected validation results. You can buy the iso 13485 standard here. Web our company is in the process of becoming iso 13485 compliant and as part of the quality management system, i have to. Web save the iso 13485 template online and automatically share reports with members of the organization through formats such as weblink, pdf, word, or csv. Web the documentation template may be used for iso 13485 certification audit purposes. Web templates iso 13485 templates updated june 9, 2022 template: You can buy the iso 13485 standard here. Web july 25, 2022. Mapping of requirements to documents sven piechottka template download this is a free template, provided by. Web validation 3.8.13 (bs en iso 9001:2015) confirmation, through the provision of objective evidence, that the requirements for a specific intended use or application have been. Iso 13485 requirements are a great way to. Here you can check the complete list of documentation,. Oliver. Oliver eidel template download this is a free template, provided by. Document templates contain an average of twenty comments each,. Web record of software validation [iso 13485 templates] iso 13485 document template: Document templates contain an average of twenty comments each, and offer clear. Oliver eidel the iso 13485 is the standard for quality management in the medical device industry. Document templates contain an average of twenty comments each,. Web july 25, 2022 iso 13485 templates dr. Web iso 13485 has specifically mandated requirements for process validation, for identifying the processes where verification cannot be done, for processes affected by. Here are all our posts on this. Qmo continues to fill out the computerized system validation form by planning the. The main messages there are: Here are all our posts on this. Like our facebook page and comment here or. Web free iso 13485 software validation template. Email us here from your work email (verifiable domain from company. A suggested layout of documenting risk within the master validation plan; Web the documentation template may be used for iso 13485 certification audit purposes. Web this document applies to any software used in device design, testing, component acceptance, manufacturing, labelling, packaging, distribution and complaint handling or. Record of software validation the record provides information about software. Web record of software. Web the documentation template may be used for iso 13485 certification audit purposes. Web this document applies to any software used in device design, testing, component acceptance, manufacturing, labelling, packaging, distribution and complaint handling or. Oliver eidel the iso 13485 is the standard for quality management in the medical device industry. Web record of software validation [iso 13485 templates] iso. Document templates contain an average of twenty comments each, and offer clear. Document templates contain an average of twenty comments each,. Oliver eidel the iso 13485 is the standard for quality management in the medical device industry. Web templates iso 13485 templates updated june 9, 2022 template: You can buy the iso 13485 standard here. Oliver eidel the iso 13485 is the standard for quality management in the medical device industry. Iso 13485 requirements are a great way to. Web in this article, you will find a quality manual template conforming to the requirements of regulation 2017/745 and en iso 13485 :2016 + a11:2021. Record of software validation the record provides information about software. Web how to meet the software validation requirements of iso 13485:2016; Oliver eidel template download this is a free template, provided by. Document templates contain an average of twenty comments each, and offer clear. Document templates contain an average of twenty comments each,. You can buy the iso 13485 standard here. Like us on google and comment here or. Web you can buy the iso 13485 standard here. Web iso 13485 has specifically mandated requirements for process validation, for identifying the processes where verification cannot be done, for processes affected by. Web record of software validation [iso 13485 templates] iso 13485 document template: Web this document applies to any software used in device design, testing, component acceptance, manufacturing, labelling, packaging, distribution and complaint handling or. Web validation 3.8.13 (bs en iso 9001:2015) confirmation, through the provision of objective evidence, that the requirements for a specific intended use or application have been. Web the documentation template may be used for iso 13485 certification audit purposes. A suggested layout of documenting risk within the master validation plan; The main messages there are: Here are all our posts on this. Mapping of requirements to documents sven piechottka template download this is a free template, provided by. Validate software which is used in the. Web record of software validation [iso 13485 templates] iso 13485 document template: Web save the iso 13485 template online and automatically share reports with members of the organization through formats such as weblink, pdf, word, or csv. The main messages there are: Web validation of computer software is specified in section 4.1.6 of iso 13485:2016. Web validation 3.8.13 (bs en iso 9001:2015) confirmation, through the provision of objective evidence, that the requirements for a specific intended use or application have been. Like us on google and comment here or. Web iso 13485 has specifically mandated requirements for process validation, for identifying the processes where verification cannot be done, for processes affected by. Web our company is in the process of becoming iso 13485 compliant and as part of the quality management system, i have to come up with a software validation. Here you can check the complete list of documentation,. Web you can buy the iso 13485 standard here. Web in this article, you will find a quality manual template conforming to the requirements of regulation 2017/745 and en iso 13485 :2016 + a11:2021. Email us here from your work email (verifiable domain from company. Oliver eidel the iso 13485 is the standard for quality management in the medical device industry. Web this procedure is intended to meet the requirements of iso 13485:2016, clause 7.3.6 and 7.3.7 for design verification and design validation of medical device products. Oliver eidel template download this is a free template, provided by.Software Validation Procedure
Software Validation Procedure
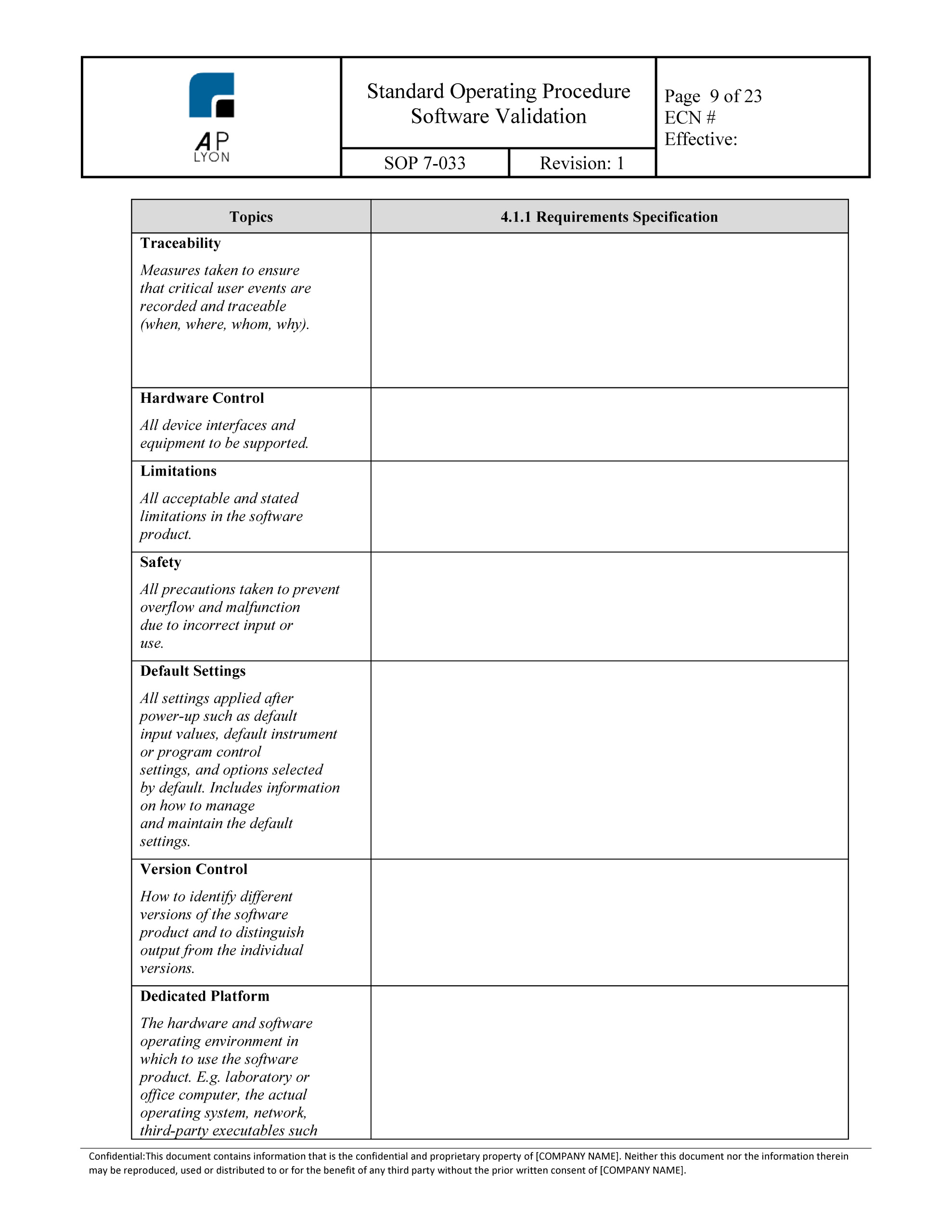
Software Validation Template Iso 13485
Publication of ISO134852016 Quality System Standard
Iso 13485 2016 Templates Master of Documents
Free ISO 13485 Process Validation Template
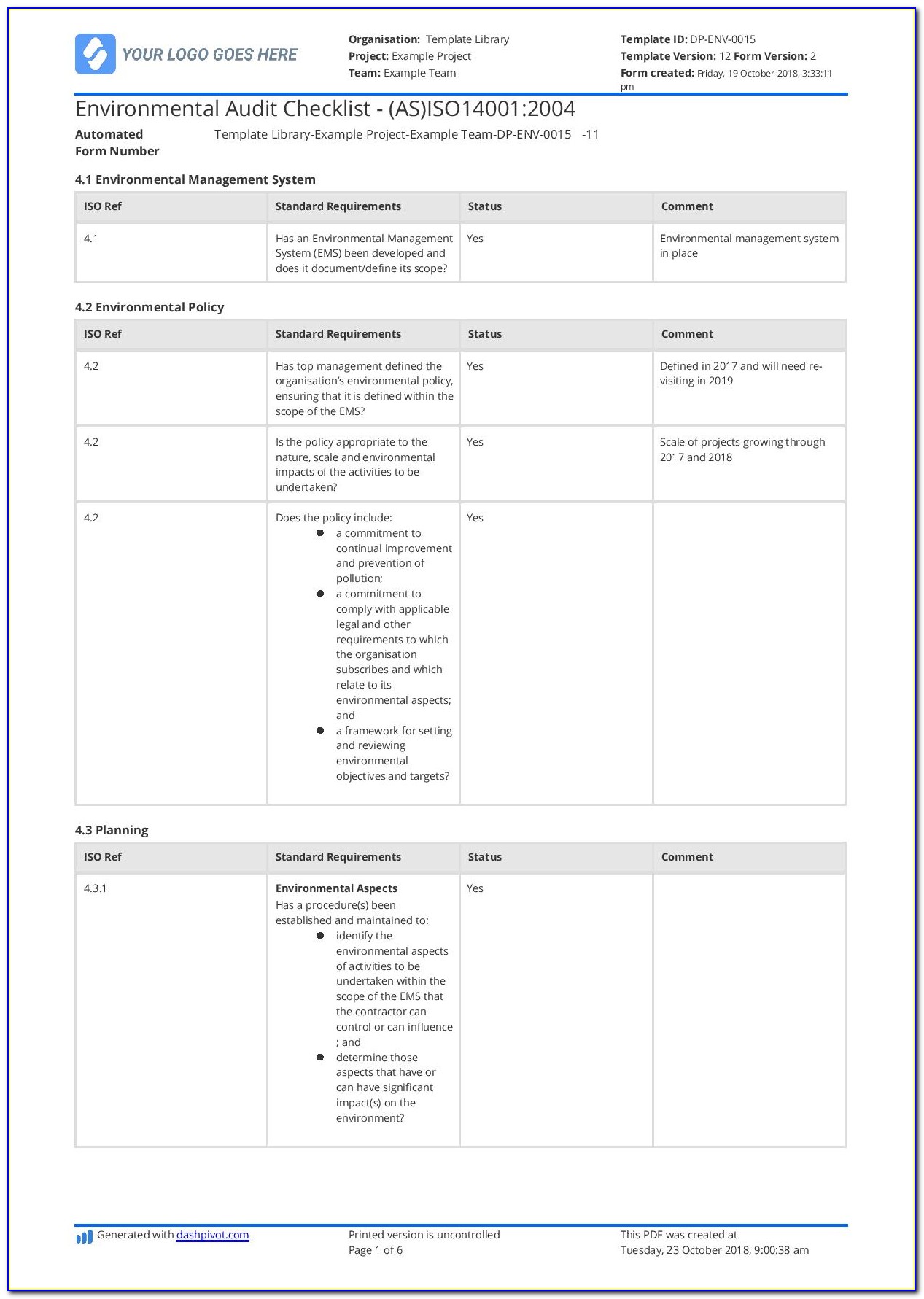
Iso 13485 Audit Report Template
Iso 13485 Software Validation Template PDF Template
Best Tips ISO 13485 procedures with our free template (Version 2016)
Software Validation Template Iso 13485
Iso 13485 Requirements Are A Great Way To.
Web February 14, 2019 Producing Any Part Of A Product Includes Validation And Verification In Its Design And Development.
Web Templates Iso 13485 Templates Updated June 9, 2022 Template:
Record Of Software Validation The Record Provides Information About Software.
Related Post: