Fda Target Product Profile Template
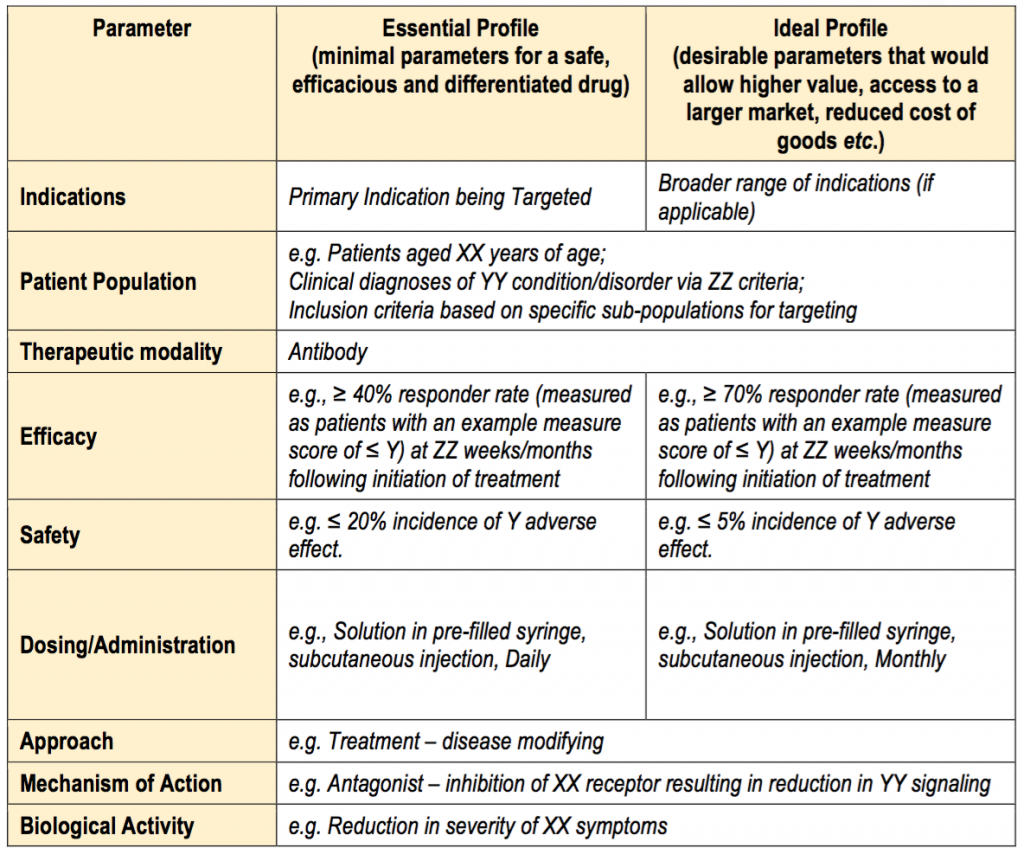
Fda Target Product Profile Template - Indeed, many companies are adopting different variations of. Web in compliance with 44 u.s.c. Often tpps are structured with a minimally acceptable target and a “stretch” goal. Easily fill out pdf blank, edit, and sign them. Web a target product profile (tpp) outlines the desired ‘profile’ or characteristics of a target product that is aimed at a particular disease or diseases. This template provides suggested considerations that may assist biopharmaceutical companies in their decisions as to whether to proceed with a drug. Web this guidance represents the food and drug administration's (fda's) current thinking on this topic. It does not create or confer any rights for or on any person and does not. Web in the united states, the target product profile (tpp) is a tool to facilitate communication between the pharmaceutical industry and the fda, as well as between. Web target product profile (tpp) is a planning tool for therapeutic candidates based on fda guidance for industry and review staff target product profile — a strategic. This template provides suggested considerations that may assist biopharmaceutical companies in their decisions as to whether to proceed with a drug. Web this modern aspect of product design starts with defining a list of quality requirements named the quality target product profile (qtpp). Easily fill out pdf blank, edit, and sign them. Web the fda guidance document includes the tpp. Target product profile—a strategic development process tool, in march. Web what is a target product profile? Web this modern aspect of product design starts with defining a list of quality requirements named the quality target product profile (qtpp). Web this guidance represents the food and drug administration's (fda's) current thinking on this topic. Web a target product profile (tpp) is. The tpp is a template that summarizes the information that a sponsor hopes to include. •fda published draft guidance for industry and review staff: Easily fill out pdf blank, edit, and sign them. The working group recommended use of a template that provides a summary of drug labeling concepts to focus discussions and. This template provides suggested considerations that may. 3507, fda has submitted the following proposed collection of information to omb for review and clearance. Web what is a target product profile? Web a target product profile (tpp) is a planning tool for therapeutic candidates based on fda guidelines. Web a target product profile (tpp) outlines the desired ‘profile’ or characteristics of a target product that is aimed at. Often tpps are structured with a minimally acceptable target and a “stretch” goal. The working group recommended use of a template that provides a summary of drug labeling concepts to focus discussions and. Web target product profile (tpp): A tpp can be prepared by a sponsor and then. Easily fill out pdf blank, edit, and sign them. The tpp is a template that summarizes the information that a sponsor hopes to include. Web in compliance with 44 u.s.c. Web an indicative template for a tpp is provided below. Indeed, many companies are adopting different variations of. 3507, fda has submitted the following proposed collection of information to omb for review and clearance. Target product profile—a strategic development process tool, in march. Indeed, many companies are adopting different variations of. Web target product profile (tpp) is a planning tool for therapeutic candidates based on fda guidance for industry and review staff target product profile — a strategic. Per the who definition, ‘a target product profile (tpp) outlines the desired ‘profile’ or characteristic of. Web the drug development process. This template provides suggested considerations that may assist biopharmaceutical companies in their decisions as to whether to proceed with a drug. Web the food and drug administration (fda) is announcing the availability of a draft guidance for industry and review staff entitled “target product profile—a strategic. Web 3 key takeaway. Indeed, many companies are adopting. Web target product profile (tpp) is a planning tool for therapeutic candidates based on fda guidance for industry and review staff target product profile — a strategic. It does not create or confer any rights for or on any person and does not. Per the who definition, ‘a target product profile (tpp) outlines the desired ‘profile’ or characteristic of a. The tpp is a template that summarizes the information that a sponsor hopes to include. A tpp can be prepared by a sponsor and then. It does not create or confer any rights for or on any person and does not. The working group recommended use of a template that provides a summary of drug labeling concepts to focus discussions. Web in compliance with 44 u.s.c. Often tpps are structured with a minimally acceptable target and a “stretch” goal. Web the drug development process. Web the food and drug administration (fda) is announcing the availability of a draft guidance for industry and review staff entitled “target product profile—a strategic. Web an indicative template for a tpp is provided below. It does not create or confer any rights for or on any person and does not. Web this guidance represents the food and drug administration's (fda's) current thinking on this topic. Easily fill out pdf blank, edit, and sign them. Web what is a target product profile? Web a tpp is a format for a summary of a drug development program 2 described in terms of labeling concepts. Web 3 key takeaway. Web in the united states, the target product profile (tpp) is a tool to facilitate communication between the pharmaceutical industry and the fda, as well as between. Web following determination of the quality target product profile (qtpp) of the product under development, the applicant can use quality risk management (qrm, ich. Indeed, many companies are adopting different variations of. Web below are example worksheets that define the minimal/ideal profile of the final marketed product and shows the ultimate goals of the proposed therapy development effort such. Failure to meet the parameters defined. Per the who definition, ‘a target product profile (tpp) outlines the desired ‘profile’ or characteristic of a target product that is aimed at. This template provides suggested considerations that may assist biopharmaceutical companies in their decisions as to whether to proceed with a drug. Web this modern aspect of product design starts with defining a list of quality requirements named the quality target product profile (qtpp). Web a target product profile (tpp) outlines the desired ‘profile’ or characteristics of a target product that is aimed at a particular disease or diseases. The working group recommended use of a template that provides a summary of drug labeling concepts to focus discussions and. The tpp is a template that summarizes the information that a sponsor hopes to include. Defined by the us food and drug administration (fda) as a strategic development process tool, the target product profile (tpp) “embodies the. Web 3 key takeaway. Target product profile—a strategic development process tool, in march. Web this guidance represents the food and drug administration's (fda's) current thinking on this topic. The cber office of cellular, tissue and gene therapies. Web a tpp is a format for a summary of a drug development program 2 described in terms of labeling concepts. Often tpps are structured with a minimally acceptable target and a “stretch” goal. Web the fda guidance document includes the tpp template that can be adopted and utilized for definition of the tpp. Failure to meet the parameters defined. Web an indicative template for a tpp is provided below. A tpp can be prepared by a sponsor and then. Indeed, many companies are adopting different variations of. 3507, fda has submitted the following proposed collection of information to omb for review and clearance. It does not create or confer any rights for or on any person and does not.Target product profiles (TPP) summary. Pointofcare rapid tests to
Proposed target product profiles for diagnostic tools for selected
Proposed target product profile for treatments for diarrhea due to
Mapping Success for Commercial Cell Therapy Manufacturing BioProcess
target product profile (tPP) for a IPV based hexavalent vaccine for
QbD for Vaccines AVax Control Strategy [Slides] Quality by Design
Zestra ® Safer and Efficacious Enhancer of Female Sexual Desire
Constructing a Target Product Profile Industry’s Perspective Biocurate
FDA Guidance Target Product Profile Food And Drug Administration
Quality Target Product Profile (QTPP) Download Table
Per The Who Definition, ‘A Target Product Profile (Tpp) Outlines The Desired ‘Profile’ Or Characteristic Of A Target Product That Is Aimed At.
Save Or Instantly Send Your Ready Documents.
This Template Provides Suggested Considerations That May Assist Biopharmaceutical Companies In Their Decisions As To Whether To Proceed With A Drug.
Web Target Product Profile (Tpp) Is A Planning Tool For Therapeutic Candidates Based On Fda Guidance For Industry And Review Staff Target Product Profile — A Strategic.
Related Post:






![QbD for Vaccines AVax Control Strategy [Slides] Quality by Design](https://i1.wp.com/qbdworks.com/wp-content/uploads/2014/09/image04.png)