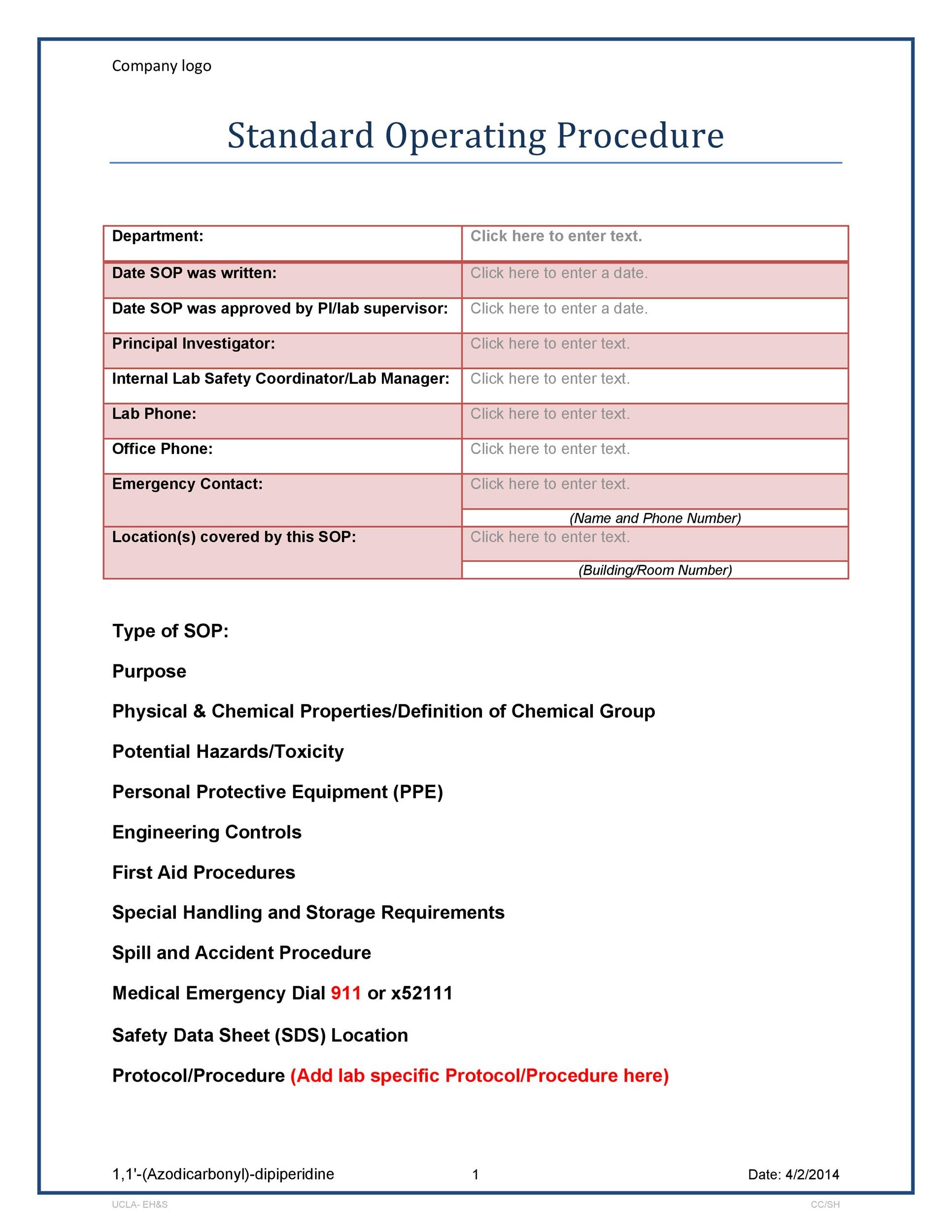
Fda Sop Template
Fda Sop Template - Web complete fda sop template online with us legal forms. The sops should describe a process for identifying suspect product. Creating and maintaining sops does not need to be a. We often hear the acronym sop and usually associate it with. Web here is a sample fda software validation template: Drug listing regulations in 1972,. Easily fill out pdf blank, edit, and sign them. Approved by date [signature] , principal investigator [signature]. Web inspection preparedness standard operating procedure template sop number: Any optional section that does not have content will be annotated with “not applicable” or “none”. Web it is important to note the fda has no requirement for what constitutes an sop or how it should be formatted, yet one of the first items a consultant or fda auditor will request is. We often hear the acronym sop and usually associate it with. Web food and drug administration office of regulatory affairs ora laboratory manual volume. Web latex template for fda sop (standard operating procedure) is there a template that could help me formatting look like an official fda sop? Drug listing regulations in 1972,. Purpose/policy this document describes the medical device single audit program (mdsap) procedures to develop, review, approve,. Equipment identification and records a. Web fda sends warning letters to companies for not having. Web complete fda sop template online with us legal forms. The sops should describe a process for identifying suspect product. Creating and maintaining sops does not need to be a. Outlines the scope of the validation project and the strategy for validating the software’s. 12/11/2019 ora establishment registration and control procedure (formerly fmd# 92) page 1 of 29. Web food and drug administration office of regulatory affairs ora laboratory manual volume ii document number: The compliance monitoring team has created standard operating procedure templates (sops) in response to. Outlines the scope of the validation project and the strategy for validating the software’s. Purpose/policy this document describes the medical device single audit program (mdsap) procedures to develop, review, approve,.. Web latex template for fda sop (standard operating procedure) is there a template that could help me formatting look like an official fda sop? Purpose/policy this document describes the medical device single audit program (mdsap) procedures to develop, review, approve,. Web it is important to note the fda has no requirement for what constitutes an sop or how it should. Web food safety plan & haccp templates. Web this manual of policies and procedures (mapp) specifies the factors to consider when determining whether to develop a mapp or a standard operating. All equipment in the fda equipment. 12/11/2019 ora establishment registration and control procedure (formerly fmd# 92) page 1 of 29. The sops should describe a process for investigating suspect. Web complete fda sop template online with us legal forms. Purpose/policy this document describes the medical device single audit program (mdsap) procedures to develop, review, approve,. Web it is important to note the fda has no requirement for what constitutes an sop or how it should be formatted, yet one of the first items a consultant or fda auditor will. Food and drug administration's (fda) food safety plan builder (fspb) is a tool designed to assist owners/operators of food facilities with the development of food safety. Save or instantly send your ready documents. Easily fill out pdf blank, edit, and sign them. Any optional section that does not have content will be annotated with “not applicable” or “none”. We often. Save or instantly send your ready documents. Web sops and sop templates are also common in government and military organizations. Procedures describe who, what, when, and how. Any optional section that does not have content will be annotated with “not applicable” or “none”. Web here is a sample fda software validation template: Web inspection preparedness standard operating procedure template sop number: Outlines the scope of the validation project and the strategy for validating the software’s. Save or instantly send your ready documents. The compliance monitoring team has created standard operating procedure templates (sops) in response to. Fda staff manual guide (smg) 2620.2, procedure for surplus equipment 6. All equipment in the fda equipment. Web inspection preparedness standard operating procedure template sop number: Food and drug administration's (fda) food safety plan builder (fspb) is a tool designed to assist owners/operators of food facilities with the development of food safety. The sops should describe a process for identifying suspect product. Fda staff manual guide (smg) 2620.2, procedure for surplus equipment 6. Creating and maintaining sops does not need to be a. Use sops to show compliance with some of the following standards and. Approved by date [signature] , principal investigator [signature]. Web latex template for fda sop (standard operating procedure) is there a template that could help me formatting look like an official fda sop? Drug listing regulations in 1972,. We often hear the acronym sop and usually associate it with. Web here is a sample fda software validation template: Purpose/policy this document describes the medical device single audit program (mdsap) procedures to develop, review, approve,. Web sops and sop templates are also common in government and military organizations. Web download our free and premium templates for useful guidance and to make work much easier. Web food and drug administration office of regulatory affairs ora laboratory manual volume ii document number: Web complete fda sop template online with us legal forms. Any optional section that does not have content will be annotated with “not applicable” or “none”. Web 30 free sop templates [word] (standard operating procedure) march 28, 2021 9 mins read. Web it is important to note the fda has no requirement for what constitutes an sop or how it should be formatted, yet one of the first items a consultant or fda auditor will request is. Use sops to show compliance with some of the following standards and. Web latex template for fda sop (standard operating procedure) is there a template that could help me formatting look like an official fda sop? Web inspection preparedness standard operating procedure template sop number: Any optional section that does not have content will be annotated with “not applicable” or “none”. Web here is a sample fda software validation template: Web complete fda sop template online with us legal forms. We often hear the acronym sop and usually associate it with. Web download our free and premium templates for useful guidance and to make work much easier. The sops should describe a process for investigating suspect product that has been detected/identified,. Creating and maintaining sops does not need to be a. Save or instantly send your ready documents. Web 30 free sop templates [word] (standard operating procedure) march 28, 2021 9 mins read. Drug listing regulations in 1972,. Procedures describe who, what, when, and how. Our professionally designed templates are guaranteed to make you draft an. The sops should describe a process for identifying suspect product.How to write a standard operating procedure sop
Operating Procedure Example Sample Templates Standard FDA Sop inside
Army Sop Template
PRODUCT RECALLS SOP Template PH32 GMP, QSR & ISO Compliance
Fda Recall Plan Template Fresh Fda Responds to Failures In Recall
SOP For Corrective Action and Preventive Actions Pharmaceutical
37 Best Standard Operating Procedure (SOP) Templates
Complaint Handling Procedure
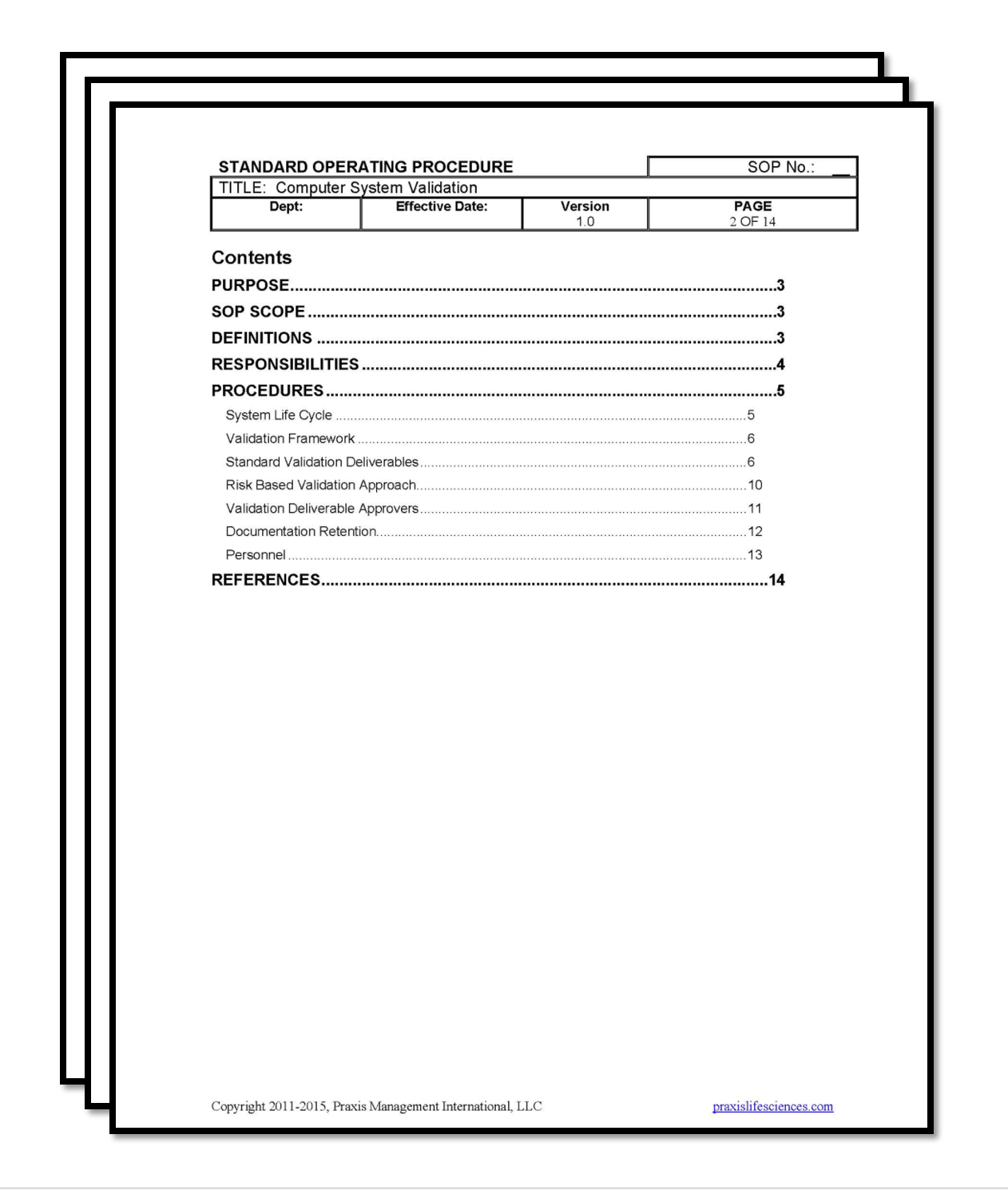
Computer System Validation SOP Validation Center
21 CFR Part 11 Assessment Template
Web It Is Important To Note The Fda Has No Requirement For What Constitutes An Sop Or How It Should Be Formatted, Yet One Of The First Items A Consultant Or Fda Auditor Will Request Is.
Approved By Date [Signature] , Principal Investigator [Signature].
Purpose/Policy This Document Describes The Medical Device Single Audit Program (Mdsap) Procedures To Develop, Review, Approve,.
All Equipment In The Fda Equipment.
Related Post: