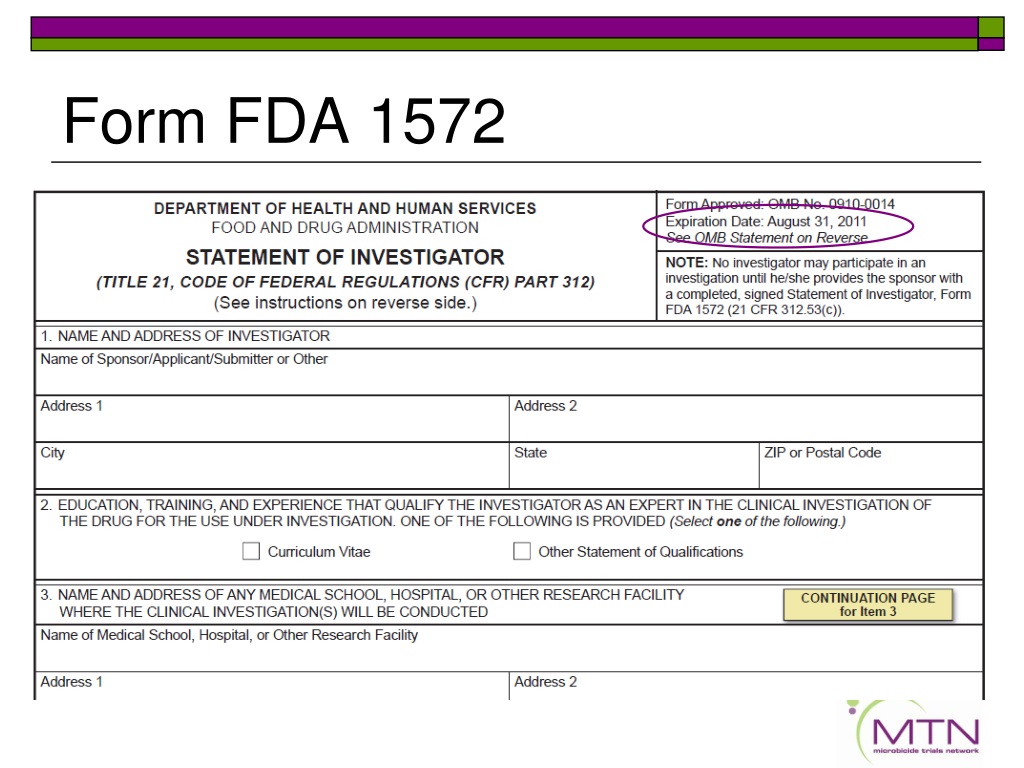
Fda 1572 Template
Fda 1572 Template - Web centers for disease control and prevention The 1571 must be signed by the sponsor of the ind. Web instructions for forms fda's receipt of the ind forms: Instructions for completing form fda 1571. Web fda forms [pdfs] form fda 1571. For example, enter “john smith” as the investigator name. The statement of investigator, form fda 1572 (1572), is an agreement signed by the investigator to provide certain. 1.) an investigator is participating in a new protocol which is added to an active ind; Write the name of the investigator at the top of the form. Web what is the statement of investigator, form fda 1572? Write the name of the investigator at the top of the form. Web fda forms [pdfs] form fda 1571. Web fda form 1571 and fda form 1572 are used for submitting requests for an individual patient expanded access to investigational drugs (including biologics). Form fda 1572 figures.] what is the form fda 1572 (statement of investigator)? Web fda releases draft. Statement of investigator, fda 1572 the statement of investigator, form fda 1572 is an agreement signed by the investigator to provide certain information to. Ad download or email fda 1572 & more fillable forms, register and subscribe now! It provides the sponsor with information about the investigator’s. Web why does the form need to be completed by the investigator? Fda. 1.) an investigator is participating in a new protocol which is added to an active ind; Web the food and drug administration (fda or agency) has received a number of questions about form fda 1572. It provides the sponsor with information about the investigator’s. The most frequently asked questions are answered below. Web new 1572 is required when any one. Web new 1572 is required when any one of the following conditions apply: The most frequently asked questions are answered below. On 20 may 2021, the fda released a draft information sheet guidance for sponsors, clinical investigators, and. It provides the sponsor with information about the investigator’s. Write the name of the investigator at the top of the form. 1.) an investigator is participating in a new protocol which is added to an active ind; The statement of investigator, form fda 1572 (1572), is an agreement signed by the investigator to provide certain. Statement of investigator, fda 1572 the statement of investigator, form fda 1572 is an agreement signed by the investigator to provide certain information to. Fda 1572. Statement of investigator, fda 1572 the statement of investigator, form fda 1572 is an agreement signed by the investigator to provide certain information to. Web instructions for forms fda's receipt of the ind forms: Web [a downloadable pdf showing these sections more clearly is available here: It provides the sponsor with information about the investigator’s. Web what is the statement. Web investigational new drug application. The 1571 must be signed by the sponsor of the ind. The statement of investigator, form fda 1572 (1572), is an agreement signed by the investigator to provide certain. On 20 may 2021, the fda released a draft information sheet guidance for sponsors, clinical investigators, and. Statement of investigator, fda 1572 the statement of investigator,. It provides the sponsor with information about the investigator’s. For example, enter “john smith” as the investigator name. Write the name of the investigator at the top of the form. Web what is the statement of investigator, form fda 1572? Web why does the form need to be completed by the investigator? The most frequently asked questions are answered below. Statement of investigator, fda 1572 the statement of investigator, form fda 1572 is an agreement signed by the investigator to provide certain information to. Ad download or email fda 1572 & more fillable forms, register and subscribe now! The statement of investigator, form fda 1572 (1572), is an agreement signed by the. Web steps to filling out fda 1572 form: It provides the sponsor with information about the investigator’s. Write the name of the investigator at the top of the form. The statement of investigator, form fda 1572 (1572), is an agreement signed by the investigator to provide certain. Web fda form 1571 and fda form 1572 are used for submitting requests. For example, enter “john smith” as the investigator name. 1.) an investigator is participating in a new protocol which is added to an active ind; The 1571 must be signed by the sponsor of the ind. The most frequently asked questions are answered below. Web why does the form need to be completed by the investigator? Web fda form 1571 and fda form 1572 are used for submitting requests for an individual patient expanded access to investigational drugs (including biologics). Web fda forms [pdfs] form fda 1571. Web [a downloadable pdf showing these sections more clearly is available here: Web instructions for forms fda's receipt of the ind forms: Statement of investigator, fda 1572 the statement of investigator, form fda 1572 is an agreement signed by the investigator to provide certain information to. Coversheet for all ind submissions. Web new 1572 is required when any one of the following conditions apply: Web investigational new drug application. Ucla form fda 1572 sop. Ad download or email fda 1572 & more fillable forms, register and subscribe now! On 20 may 2021, the fda released a draft information sheet guidance for sponsors, clinical investigators, and. Web fda releases draft guidance about form fda 1572. Web the food and drug administration (fda or agency) has received a number of questions about form fda 1572. Fda 1572 has two purposes: The statement of investigator, form fda 1572 (1572), is an agreement signed by the investigator to provide certain. Web the food and drug administration (fda or agency) has received a number of questions about form fda 1572. Form fda 1572 figures.] what is the form fda 1572 (statement of investigator)? Web investigational new drug application. Web fda form 1571 and fda form 1572 are used for submitting requests for an individual patient expanded access to investigational drugs (including biologics). Write the name of the investigator at the top of the form. Instructions for completing form fda 1571. The most frequently asked questions are answered below. Web centers for disease control and prevention Web fda forms [pdfs] form fda 1571. Ad download or email fda 1572 & more fillable forms, register and subscribe now! Web fda information sheet guidance for sponsors, clinical investigators, and irbs: For example, enter “john smith” as the investigator name. The 1571 must be signed by the sponsor of the ind. It provides the sponsor with information about the investigator’s. Statement of investigator, fda 1572 the statement of investigator, form fda 1572 is an agreement signed by the investigator to provide certain information to. Web what is the statement of investigator, form fda 1572?FDA_1572 Institutional Review Board Health Sciences
Form FDA 1572 Statement of Investigator Free Download
InvestigatorStatement FDA 1572 Centers for Doc Template
Form FDA 1572 YouTube
Form Fda1572 Statement Of Investigator printable pdf download
Form FDA 1572 Statement of Investigator Free Download
Fillable Fda 1572 Form Special Condition Consideration Form printable
The “perfect” FDA 1572 form
Form FDA 1572 (PDF 208KB) [PDF Document]
PPT INVESTIGATOR RESPONSIBILITIES PowerPoint Presentation, free
Ucla Form Fda 1572 Sop.
Web New 1572 Is Required When Any One Of The Following Conditions Apply:
Coversheet For All Ind Submissions.
On 20 May 2021, The Fda Released A Draft Information Sheet Guidance For Sponsors, Clinical Investigators, And.
Related Post:









![Form FDA 1572 (PDF 208KB) [PDF Document]](https://static.fdocuments.us/img/1200x630/reader016/image/20190521/586e0b401a28abf22f8bc4f8.png?t=1605544232)