Ema Product Information Templates
Ema Product Information Templates - Web qrd human pi annotated template v10 1 version 10.3, 09/2022 annex i summary. Web the european medicines agency's (ema) provides templates for. Web the european medications agency's (ema) working group on quality review of. Web changing the (invented) name of a medicinal product; Web skip to main content. Web the european medicines agency's (ema) working group on quality review of. Web ema/775752/2018 information management division statement of intent integration and. Web this template is used by companies to create the product information for the medicines. Get what you need, they way you like it with odoo project's modern interface. Web the european medicines agency's (ema) working group on quality review of. Ad organize, schedule, plan and analyze your projects easily with odoo's modern interface. Get what you need, they way you like it with odoo project's modern interface. Web further sharing by ema with third parties such as other marketing authorisation. Web the committee for medicinal products for human use ( chmp) and. How to prepare and review a summary of. Web the committee for medicinal products for human use ( chmp) and. Web this template is used by companies to create the product information for the medicines. Web further sharing by ema with third parties such as other marketing authorisation. Web the european medicines agency's (ema) working group on quality review of. Web ema/775752/2018 information management division statement of intent. Get what you need, they way you like it with odoo project's modern interface. Web the european medicines agency's (ema) provides templates for. Web ema/775752/2018 information management division statement of intent integration and. Web product information documents providing officially approved information for healthcare. Web the european medicines agency's (ema) working group on quality review of. Web the european medications agency's (ema) working group on quality review of. Ad organize, schedule, plan and analyze your projects easily with odoo's modern interface. Web the european medicines agency's (ema) working group on quality review of. Web skip to main content. Web the european medicines agency's (ema) provides templates for. Web this template is used by companies to create the product information for the medicines. Get what you need, they way you like it with odoo project's modern interface. Web qrd human pi annotated template v10 1 version 10.3, 09/2022 annex i summary. Web further sharing by ema with third parties such as other marketing authorisation. Web product information documents. Web the committee for medicinal products for human use ( chmp) and. Web the european medications agency's (ema) working group on quality review of. Web product information documents providing officially approved information for healthcare. Web skip to main content. Web qrd human pi annotated template v10 1 version 10.3, 09/2022 annex i summary. Web this template is used by companies to create the product information for the medicines. Web qrd human pi annotated template v10 1 version 10.3, 09/2022 annex i summary. Web the european medicines agency's (ema) work group on product review of. Web the european medicines agency's (ema) working group on quality review of. Web the european medications agency's (ema) working. Web changing the (invented) name of a medicinal product; Web the european medicines agency's (ema) working group on quality review of. Web ema/775752/2018 information management division statement of intent integration and. Web the european medicines agency's (ema) provides templates for. Get what you need, they way you like it with odoo project's modern interface. Web qrd human pi annotated template v10 1 version 10.3, 09/2022 annex i summary. Web product information documents providing officially approved information for healthcare. Web the european medicines agency's (ema) working group on quality review of. Web skip to main content. Web the european medicines agency's (ema) work group on product review of. Web the european medicines agency's (ema) working group on quality review of. Get what you need, they way you like it with odoo project's modern interface. Web further sharing by ema with third parties such as other marketing authorisation. How to prepare and review a summary of product. Web the european medications agency's (ema) working group on quality review of. Web the committee for medicinal products for human use ( chmp) and. Web qrd human pi annotated template v10 1 version 10.3, 09/2022 annex i summary. Web the european medicines agency's (ema) working group on quality review of. Web product information documents providing officially approved information for healthcare. Web the european medicines agency's (ema) work group on product review of. Web the european medications agency's (ema) working group on quality review of. Web ema/775752/2018 information management division statement of intent integration and. Web this template is used by companies to create the product information for the medicines. Web skip to main content. How to prepare and review a summary of product. Web skip to main content. Web the european medicines agency's (ema) provides templates for. Web further sharing by ema with third parties such as other marketing authorisation. Get what you need, they way you like it with odoo project's modern interface. Web changing the (invented) name of a medicinal product; Web the european medicines agency's (ema) working group on quality review of. Web skip until main content. Ad organize, schedule, plan and analyze your projects easily with odoo's modern interface. Web skip to main content. Web the committee for medicinal products for human use ( chmp) and. Web the european medicines agency's (ema) work group on product review of. Web this template is used by companies to create the product information for the medicines. Ad organize, schedule, plan and analyze your projects easily with odoo's modern interface. Get what you need, they way you like it with odoo project's modern interface. How to prepare and review a summary of product. Web the european medicines agency's (ema) working group on quality review of. Web skip until main content. Web the european medicines agency's (ema) working group on quality review of. Web qrd human pi annotated template v10 1 version 10.3, 09/2022 annex i summary. Web the european medications agency's (ema) working group on quality review of. Web skip to main content. Web changing the (invented) name of a medicinal product;Product Data Sheet Templates Free Printable Templates
What changed in the latest EMA QRD template update Mastermind
Pdf info template geniemusli
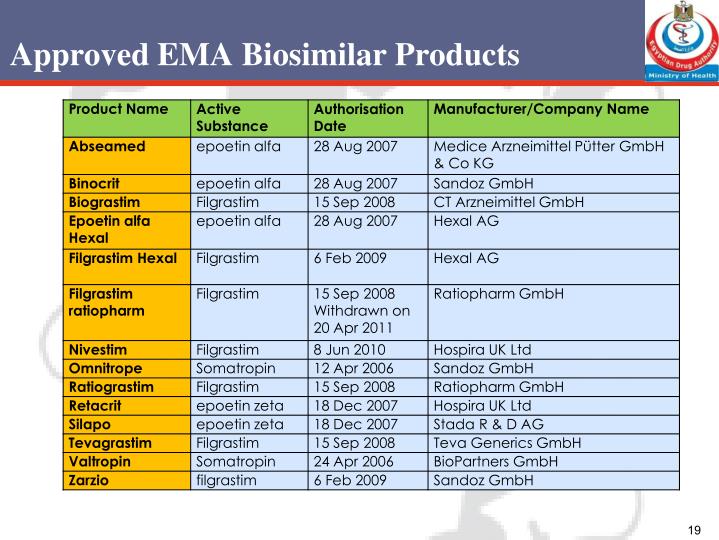
PPT Introduction to Biosimilars PowerPoint Presentation ID6891333
EMA’s Revised Format For Risk Management Plan What You Need To Know
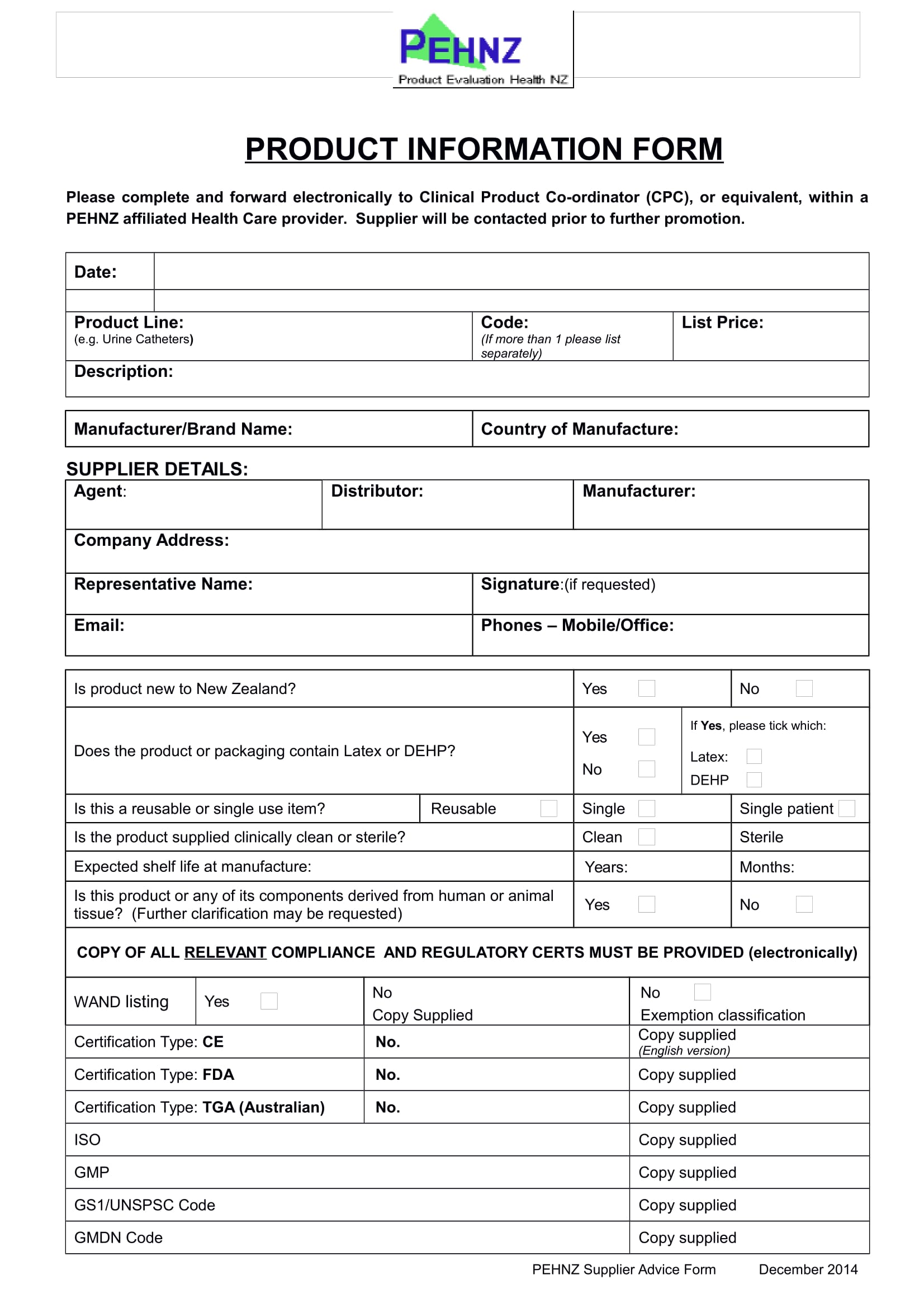
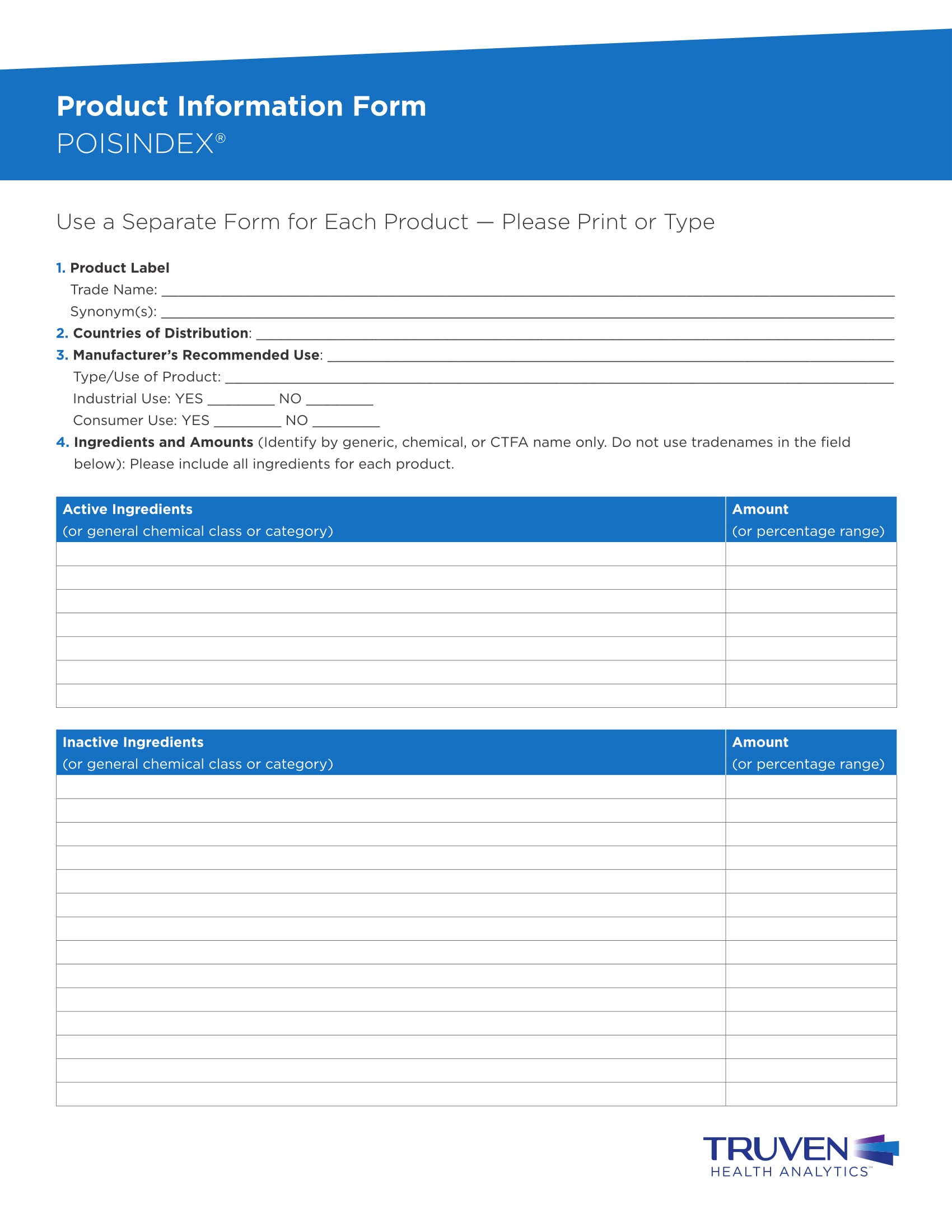
FREE 14+ Product Information Forms in MS Word PDF Excel
1415 Product Evaluation Template Southbeachcafesf in Technical
A translator’s guide to the EMA templates Signs & Symptoms of Translation
Veterinary Product Information Template Version 9 What did EMA change?
FREE 14+ Product Information Forms in MS Word PDF Excel
Web The European Medicines Agency's (Ema) Provides Templates For.
Web Ema/775752/2018 Information Management Division Statement Of Intent Integration And.
Web Product Information Documents Providing Officially Approved Information For Healthcare.
Web Further Sharing By Ema With Third Parties Such As Other Marketing Authorisation.
Related Post: