Clinical Trial Communication Plan Template
Clinical Trial Communication Plan Template - Web 3.1.3 other communication vehicles 6. This communication plan template was developed to. The final version of the communication plan template can be. Web protocol templates for clinical trials. Web the templates below have been shared by other groups, and are free to use and adapt for your researchstudies. Web 5 rows communication plan template. Web clinical trial communication plan template sponsor name clinical research communication plan for [protocol name] situation analysis write. Web none of the 48 mined clinical trial protocols integrated a communication plan. Web templates welcome to global health trials' tools and templates library. Guidance for recruitment activity log. Guidance for recruitment activity log. Web the final version of the communication plan template can be seen in the supplementary section as a pdf. Web designed to be accessible and relevant to a wide audience, communications handbook for clinical trials will make your job easier, whether you are a researcher, a study. Throughout the template there are suggested. This communication. Web clinical trials quality assurance (ctqa) regulatory templates. Purpose of communications management plan [provide. Web approach to participant communication. This communication plan should apply to all phases of a research trial, with a particular emphasis on plans to share results in an. The templates below have been shared by other groups, and are free to use and adapt for your. Web the final version of the communication plan template can be seen in the supplementary section as a pdf. Purpose of communications management plan [provide. Throughout the template there are suggested. Web in your communications plan, however, you should go beyond these general categories. Please ensure that you read and adapt them carefully for your own. Web approach to participant communication. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types. This template also includes a section for situation analysis. Throughout the template there are suggested. Web none of the 48 mined clinical trial protocols integrated a communication plan. Web develop your own communication plan using this free clinical trial communication plan template. Web approach to participant communication. Web in your communications plan, however, you should go beyond these general categories. Web instructions this template is a suggested format for a communication plan developed by tb survey teams. Web clinical trial communication plan template sponsor name clinical research communication. Guidance for recruitment activity log. Instead of defining stakeholders as “ministry of health officials,” determine specifically. Web this template aims to facilitate the development of phase 2 and 3 clinical trial protocols that require a food and drug administration (fda) investigational new drug (ind) or. Web templates welcome to global health trials' tools and templates library. This communication plan should. Web this template aims to facilitate the development of phase 2 and 3 clinical trial protocols that require a food and drug administration (fda) investigational new drug (ind) or. This communication plan template was developed to. Web templates welcome to global health trials' tools and templates library. This template also includes a section for situation analysis. The templates below have. Purpose of communications management plan [provide. Web clinical trial communication plan template sponsor name clinical research communication plan for [protocol name] situation analysis write. Documents pertinent conversations regarding the study. Please ensure that you read and adapt them carefully for your own. This communication plan template was developed to. Web none of the 48 mined clinical trial protocols integrated a communication plan. Web approach to participant communication. Web this template aims to facilitate the development of phase 2 and 3 clinical trial protocols that require a food and drug administration (fda) investigational new drug (ind) or. This communication plan should apply to all phases of a research trial, with. This communication plan template was developed to. Web instructions this template is a suggested format for a communication plan developed by tb survey teams. Successful communication methods the most suitable method of communication. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types. Documents pertinent conversations regarding the study. Instead of defining stakeholders as “ministry of health officials,” determine specifically. This communication plan template was developed to. The templates below have been shared by other groups, and are free to use and adapt for your. Purpose of communications management plan [provide. Web clinical trials quality assurance (ctqa) regulatory templates. Web instructions this template is a suggested format for a communication plan developed by tb survey teams. Please ensure that you read and adapt them carefully for your own. Web this template aims to facilitate the development of phase 2 and 3 clinical trial protocols that require a food and drug administration (fda) investigational new drug (ind) or. Successful communication methods the most suitable method of communication. We recommend that researchers utilize the developed communication. Web none of the 48 mined clinical trial protocols integrated a communication plan. Web templates welcome to global health trials' tools and templates library. This communication plan should apply to all phases of a research trial, with a particular emphasis on plans to share results in an. Web in your communications plan, however, you should go beyond these general categories. Web protocol templates for clinical trials. Web designed to be accessible and relevant to a wide audience, communications handbook for clinical trials will make your job easier, whether you are a researcher, a study. Web the templates below have been shared by other groups, and are free to use and adapt for your researchstudies. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types. Guidance for recruitment activity log. Throughout the template there are suggested. Web 5 rows communication plan template. Web this template aims to facilitate the development of phase 2 and 3 clinical trial protocols that require a food and drug administration (fda) investigational new drug (ind) or. Web none of the 48 mined clinical trial protocols integrated a communication plan. Web protocol templates for clinical trials. Documents pertinent conversations regarding the study. The final version of the communication plan template can be. They may be useful, but not required, to organize study documentation for other studies. Web instructions this template is a suggested format for a communication plan developed by tb survey teams. Web 3.1.3 other communication vehicles 6. This communication plan should apply to all phases of a research trial, with a particular emphasis on plans to share results in an. This template also includes a section for situation analysis. Please ensure that you read and adapt them carefully for your own. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types. Web approach to participant communication. Web designed to be accessible and relevant to a wide audience, communications handbook for clinical trials will make your job easier, whether you are a researcher, a study. Successful communication methods the most suitable method of communication.Free Clinical Trial Templates Smartsheet
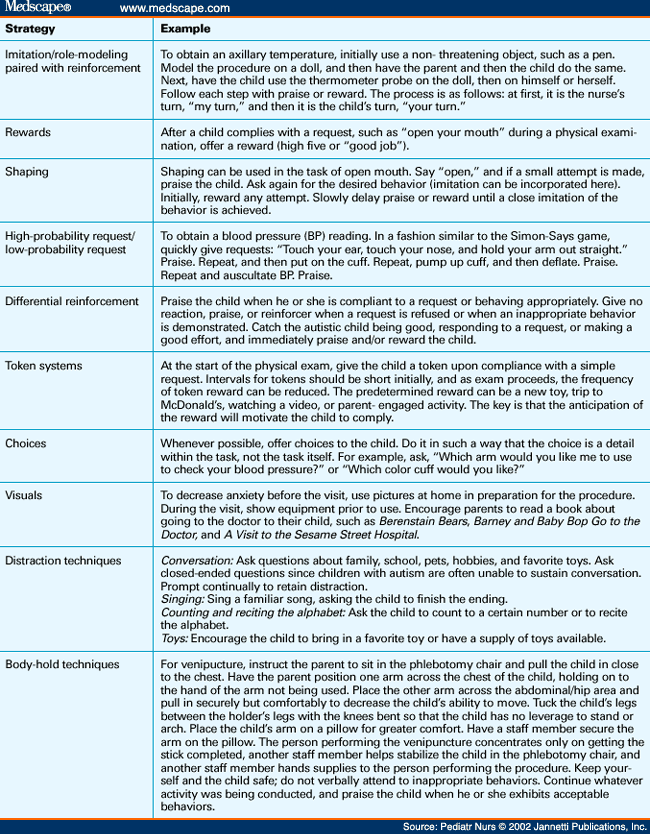
Communication Plan Communication Plan Nursing
SC Consulting » OUR WORK
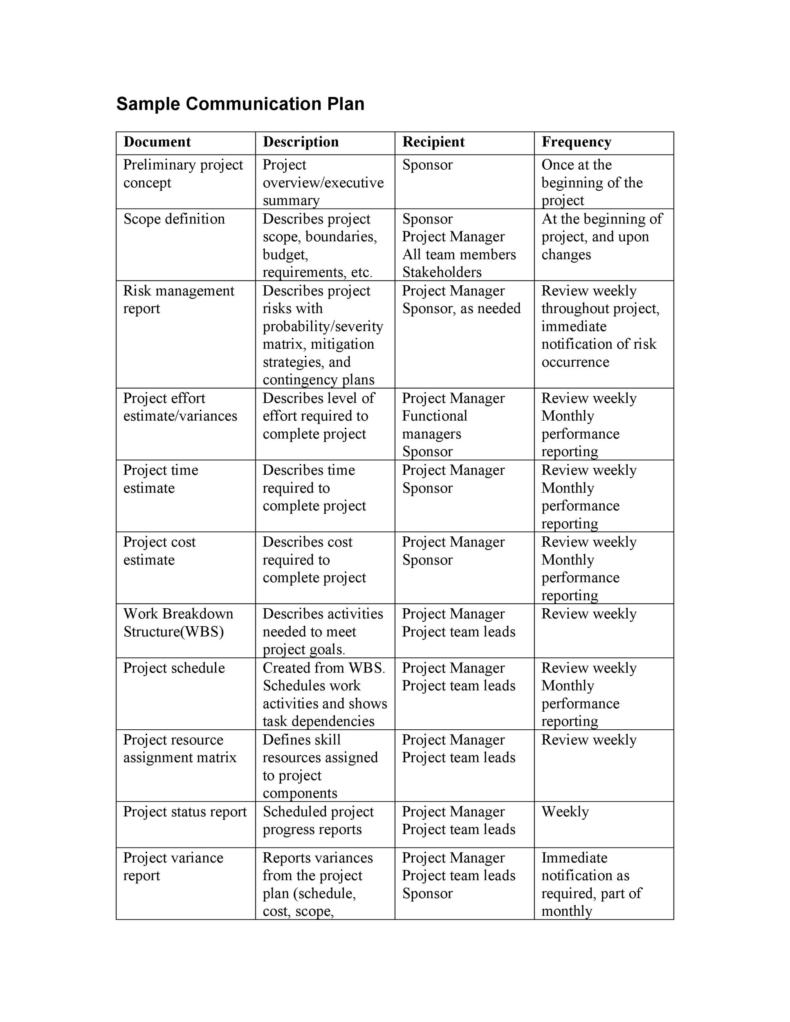
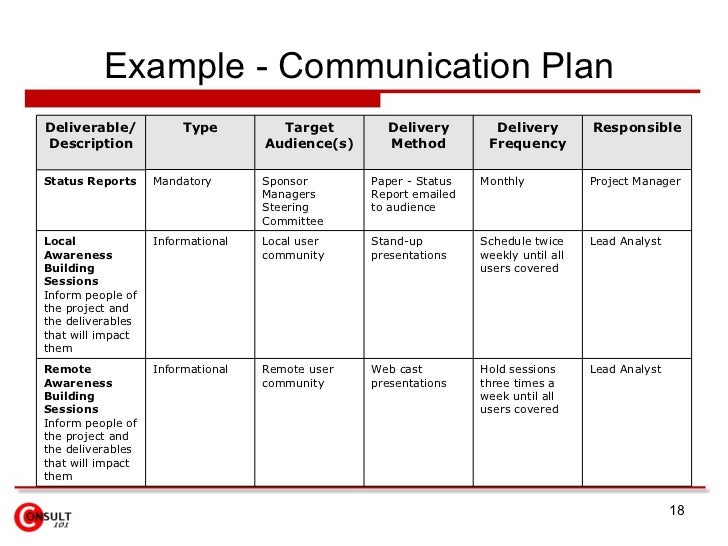
37 Simple Communication Plan Examples (+ Free Templates) ᐅ TemplateLab
Clinical Trial Protocol Template Eu Templates NjQyMjk Resume Examples
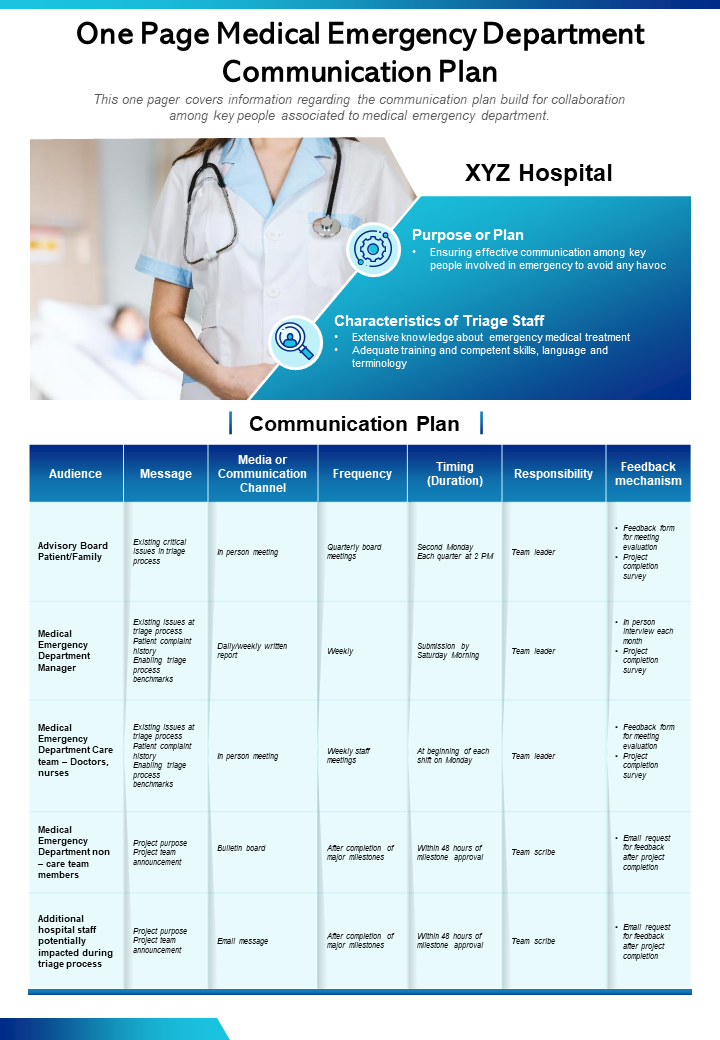
Top 10 One Page Communication Plan for Formulating Effective Business
Planning for a Communication Device Trial
The Change Management Journey of Mercy Healthcare An AIM Case Study
PBLPBLPBL TRIGGER 6
Clinical Development Plan Template Awesome Appendix C Features Of
Instead Of Defining Stakeholders As “Ministry Of Health Officials,” Determine Specifically.
Throughout The Template There Are Suggested.
The Templates Below Have Been Shared By Other Groups, And Are Free To Use And Adapt For Your.
Web Clinical Trials Quality Assurance (Ctqa) Regulatory Templates.
Related Post: