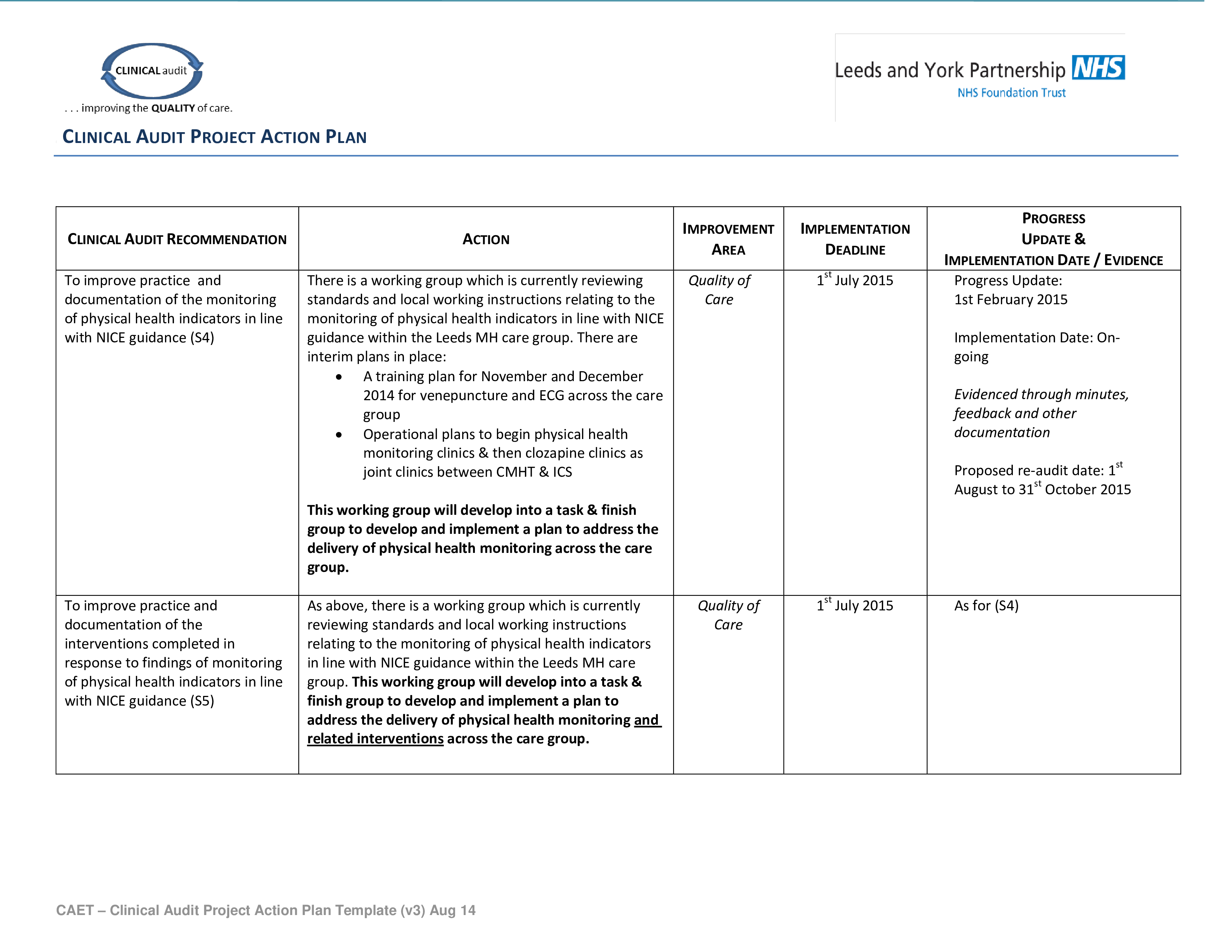
Clinical Trial Audit Plan Template
Clinical Trial Audit Plan Template - Announcement of the inspection/audit to the inspectee/auditee 3. What are we trying to achieve? Web january 16, 2020 (updated september 16, 2021) try smartsheet for free this guide provides professionals with everything they need to understand clinical data. Web an audit plan depends on several factors [1]: Web clinical study report template. Web contact us clinical trial audit checklist firms should conduct their own mock audits on a regular basis to ensure compliance and identify areas for improvement. Web trials are selected for audit per the guidelines outlined by this audit manual and the ufhcc data and safety monitoring plan (dsmp). Web clinical trial tools have been created in cooperation with the institutional review board , clinical trials audit and compliance office and the emory's office of compliance for. Web the business plan is about the clinic trial business company. Web clinical research quality is designed and embedded in the clinical trial processes and study protocol well in advance of enrollment of the first patient. Web january 16, 2020 (updated september 16, 2021) try smartsheet for free this guide provides professionals with everything they need to understand clinical data. Announcement of the inspection/audit to the inspectee/auditee 3. Web contact us clinical trial audit checklist firms should conduct their own mock audits on a regular basis to ensure compliance and identify areas for improvement. Ad simplepractice. Web trials are selected for audit per the guidelines outlined by this audit manual and the data and ufhcc safety monitoring plan (dsmp). What are we trying to achieve? Web preparation of a gcp inspection/audit 1. Web the business plan is about the clinic trial business company. Web audit plans, such as an annual plan, a monthly plan, and a. Web trials are selected for audit per the guidelines outlined by this audit manual and the data and ufhcc safety monitoring plan (dsmp). Ad simplepractice ehr for therapists now equipped with wiley treatment planners. What are we trying to achieve? Announcement of the inspection/audit to the inspectee/auditee 3. Web an audit plan depends on several factors [1]: Identification of the process(es)/outcome(s) to be. Web the announcement that an audit will be conducted in the near future always creates dread and anxiety in those persons to be visited, especially in those closest to. Web an audit plan depends on several factors [1]: Scope:what and who are to be included in the. Web trials are selected for audit per. Web the announcement that an audit will be conducted in the near future always creates dread and anxiety in those persons to be visited, especially in those closest to. Web an audit plan depends on several factors [1]: Web january 16, 2020 (updated september 16, 2021) try smartsheet for free this guide provides professionals with everything they need to understand. “plan” (preparing for the clinical audit) the planning stage is critical to the success of the audit cycle and considers: Ad simplepractice ehr for therapists now equipped with wiley treatment planners. Identification of the process(es)/outcome(s) to be. Web clinical research quality is designed and embedded in the clinical trial processes and study protocol well in advance of enrollment of the. This toolkit was initially prepared for a workshop at the. Appointment of the inspection/audit team 2. If you are in the field of pharmaceuticals and medicines and always look for users for clinical trial of medicines,. Web contact us clinical trial audit checklist firms should conduct their own mock audits on a regular basis to ensure compliance and identify areas. * trial criticality for regulatory submission. Web contact us clinical trial audit checklist firms should conduct their own mock audits on a regular basis to ensure compliance and identify areas for improvement. * the type and complexity of the trial. Budget monitoring tool with example data. * the level of risks. Web trials are selected for audit per the guidelines outlined by this audit manual and the ufhcc data and safety monitoring plan (dsmp). * the number of clinical trials. Budget monitoring tool with example data. Web trials are selected for audit per the guidelines outlined by this audit manual and the data and ufhcc safety monitoring plan (dsmp). Rights and. Web • monitoring of a clinical trial site course: Identification of the process(es)/outcome(s) to be. How to audit a clinical trial, 1 take the standard steps: Web trials are selected for audit per the guidelines outlined by this audit manual and the data and ufhcc safety monitoring plan (dsmp). Appointment of the inspection/audit team 2. Choose from 1,000+ treatment plan goals, objectives & interventions. Web clinical trials quality assurance (ctqa) regulatory templates. Web clinical trial tools have been created in cooperation with the institutional review board , clinical trials audit and compliance office and the emory's office of compliance for. Web preparation of a gcp inspection/audit 1. Ad simplepractice ehr for therapists now equipped with wiley treatment planners. Web clinical research quality is designed and embedded in the clinical trial processes and study protocol well in advance of enrollment of the first patient. Web an audit plan depends on several factors [1]: Identification of the process(es)/outcome(s) to be. Web contact us clinical trial audit checklist firms should conduct their own mock audits on a regular basis to ensure compliance and identify areas for improvement. * the type and complexity of the trial. Web trials are selected for audit per the guidelines outlined by this audit manual and the ufhcc data and safety monitoring plan (dsmp). Web the announcement that an audit will be conducted in the near future always creates dread and anxiety in those persons to be visited, especially in those closest to. Scope:what and who are to be included in the. Web it contains links to helpful references as well as templates, checklists, and forms related to fda audits and audit readiness. Budget monitoring tool with example data. “plan” (preparing for the clinical audit) the planning stage is critical to the success of the audit cycle and considers: Web overview monitoring and auditing of clinical trials is necessary to assure that the: Web trials are selected for audit per the guidelines outlined by this audit manual and the data and ufhcc safety monitoring plan (dsmp). If you are in the field of pharmaceuticals and medicines and always look for users for clinical trial of medicines,. * trial criticality for regulatory submission. Web the announcement that an audit will be conducted in the near future always creates dread and anxiety in those persons to be visited, especially in those closest to. Budget monitoring tool with example data. Ad simplepractice ehr for therapists now equipped with wiley treatment planners. Announcement of the inspection/audit to the inspectee/auditee 3. Choose from 1,000+ treatment plan goals, objectives & interventions. * the number of clinical trials. * the type and complexity of the trial. How to audit a clinical trial, 1 take the standard steps: If you are in the field of pharmaceuticals and medicines and always look for users for clinical trial of medicines,. Web preparation of a gcp inspection/audit 1. Web clinical study report template. Web contact us clinical trial audit checklist firms should conduct their own mock audits on a regular basis to ensure compliance and identify areas for improvement. Web clinical research quality is designed and embedded in the clinical trial processes and study protocol well in advance of enrollment of the first patient. Web trials are selected for audit per the guidelines outlined by this audit manual and the ufhcc data and safety monitoring plan (dsmp). Ad simplepractice ehr for therapists now equipped with wiley treatment planners. Rights and safety of patients (i.e., human subjects) are protected reported trial data are.Audit Plan Template For Clinical Trials Cards Design Templates
14+ Audit Action Plan Templates PDF
Clinical Audit Templates at
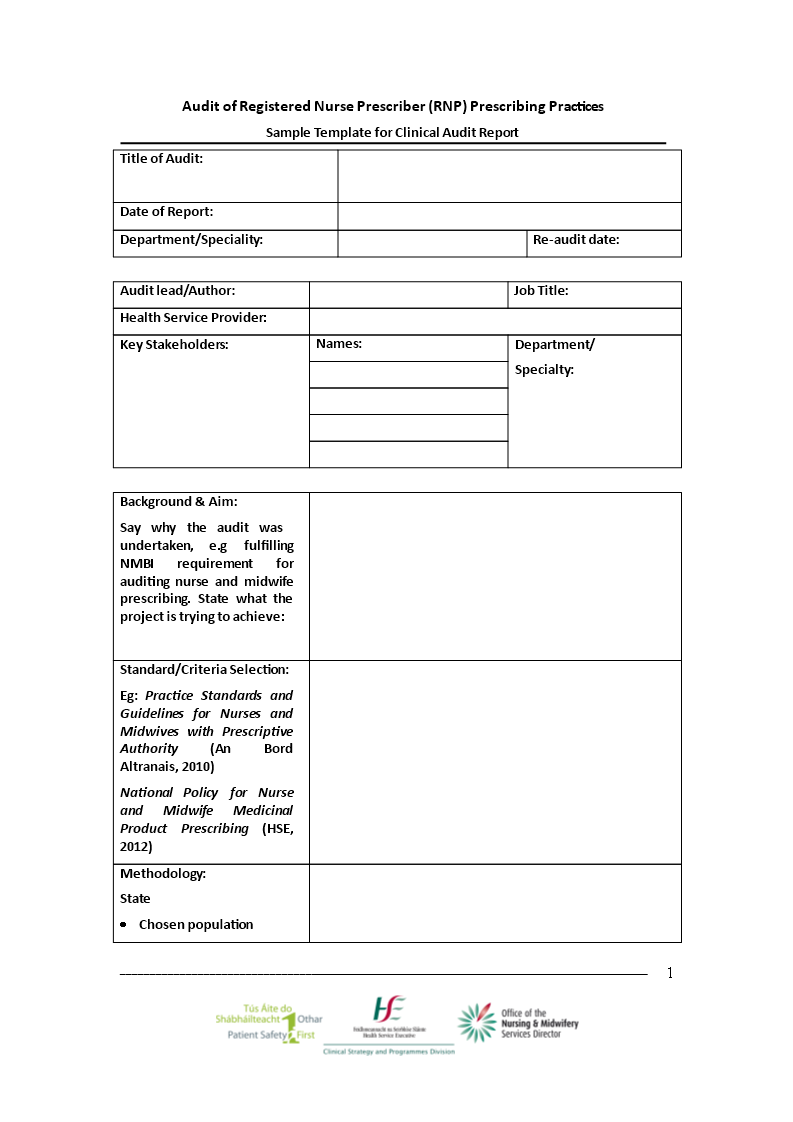
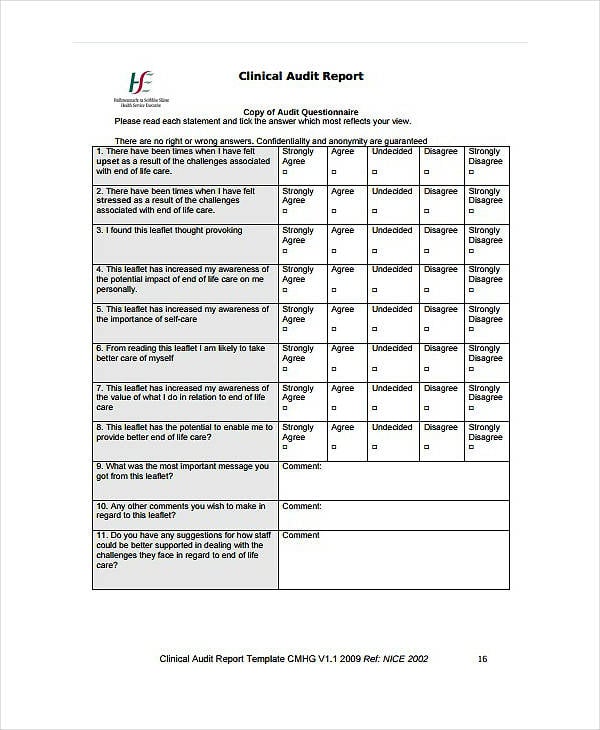
FREE 11+ Clinical Audit Report Templates in PDF MS Word
Audit Plan Template For Clinical Trials Cards Design Templates
62 Adding Audit Plan Template For Clinical Trials for Ms Word for Audit
11+ Clinical Audit Report Templates PDF, DOC
Clinical Audit Action Plan Templates at
Audit Plan Template For Clinical Trials Cards Design Templates
Free 11 Clinical Audit Report Templates In Pdf Ms Word Gambaran
Web Audit Plans, Such As An Annual Plan, A Monthly Plan, And A Plan Specific To Each Trial Or Audit, Should Be Established Based On Consideration Of The Goal (S), Contents.
Web It Contains Links To Helpful References As Well As Templates, Checklists, And Forms Related To Fda Audits And Audit Readiness.
An Example Of This Is Formatting Observations As Critical,.
Scope:what And Who Are To Be Included In The.
Related Post: