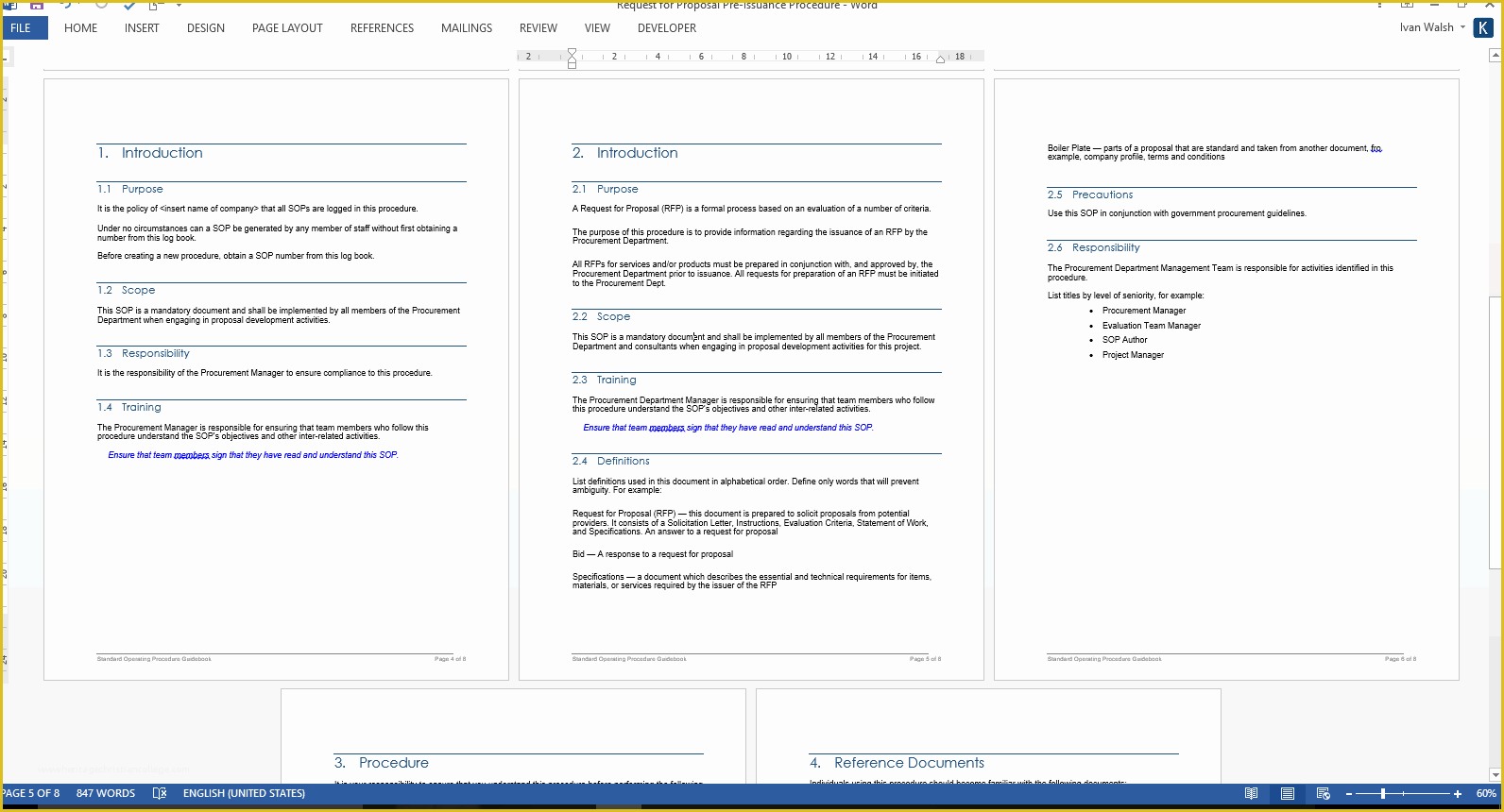
Clinical Research Sop Template
Clinical Research Sop Template - “detailed, written instructions to achieve uniformity of the performance of a specific function.” (ich gcp 1.55) in simple terms an. This template has been freely provided by. Web clinical research sops. Web in clinical research, sops help define the group’s (e.g., unit, division, department, institution, etc.) standard practices and daily processes conducted to assure. Occupational and environmental safety office (oeso) financial services. Web clickup's clinical trials sop template is designed to help you streamline your clinical trial processes and ensure compliance with standard operating procedures (sops). Web in clinical research, sops help define the group’s (e.g., unit, division, department, institution, etc.) standard practices and daily processes conducted to assure execution. Follow these six steps to create quality sops that meet ethical and regulatory standards. Web institutional policies and procedures. Web clickup's quantitative research sop template is designed to help you streamline your quantitative research processes and ensure consistency across your team. Web clickup's quantitative research sop template is designed to help you streamline your quantitative research processes and ensure consistency across your team. Web clinical research standard operating procedures. The standard operating procedures (sops) in this library are established to ensure consistency and compliance with federal/state. Via the global health network. Process for obtaining informed consent 4: Process for obtaining informed consent 4: Web learn how to write clear and consistent sops for clinical trials and research. The standard operating procedures (sops) in this library are established to ensure consistency and compliance with federal/state. Web sops are available in the areas of: Budget monitoring tool with example data. Web sops are available in the areas of: Web in clinical research, sops help define the group’s (e.g., unit, division, department, institution, etc.) standard practices and daily processes conducted to assure execution. Web clinical research standard operating procedures. Web standard operating procedures (sops) are uniformly written procedures, with detailed instructions to record routine operations, processes and practices followed. [title of. Web clinical study report template. Web clickup's clinical trials sop template is designed to help you streamline your clinical trial processes and ensure compliance with standard operating procedures (sops). Web learn how to write clear and consistent sops for clinical trials and research. Occupational and environmental safety office (oeso) financial services. The standard operating procedures (sops) in this library are. Web clinical research standard operating procedures. [title of the sop] [insert clinical research site (crs) name and crs number] [insert. List of standard operating procedures. Via the global health network. Web clickup's clinical trials sop template is designed to help you streamline your clinical trial processes and ensure compliance with standard operating procedures (sops). Web in clinical research, sops help define the group’s (e.g., unit, division, department, institution, etc.) standard practices and daily processes conducted to assure execution. Follow these six steps to create quality sops that meet ethical and regulatory standards. Web clinical research standard operating procedures. Table of contents, glossary & abbreviations. Web institutional policies and procedures. Web sops are available in the areas of: Web below are the standard operating procedures of the clinical research center. Web what are standard operating procedures (sop)? Web in clinical research, sops help define the group’s (e.g., unit, division, department, institution, etc.) standard practices and daily processes conducted to assure execution. Web community health network office of research administration sops. This template has been freely provided by. Web institutional policies and procedures. Web what are standard operating procedures (sop)? “detailed, written instructions to achieve uniformity of the performance of a specific function.” (ich gcp 1.55) in simple terms an. Follow these six steps to create quality sops that meet ethical and regulatory standards. List of standard operating procedures. Web in clinical research, sops help define the group’s (e.g., unit, division, department, institution, etc.) standard practices and daily processes conducted to assure. Web ighid clinical research acronyms and definitions template; Web what are standard operating procedures (sop)? Web standard operating procedures (sops) are uniformly written procedures, with detailed instructions to record routine operations, processes. This template has been freely provided by. Clinical research standard operating procedures. Web clinical study report template. Web ighid clinical research acronyms and definitions template; Web in clinical research, sops help define the group’s (e.g., unit, division, department, institution, etc.) standard practices and daily processes conducted to assure execution. They are categorized into 4 sections, study management, clinical operations, administrative, and. Clinical research standard operating procedures. Web below are the standard operating procedures of the clinical research center. An sop is the principal document, which describes what is to be done, who is responsible. “detailed, written instructions to achieve uniformity of the performance of a specific function.” (ich gcp 1.55) in simple terms an. Web clinical research sops. Web clickup's clinical trials sop template is designed to help you streamline your clinical trial processes and ensure compliance with standard operating procedures (sops). Web standard operating procedures (sops) are uniformly written procedures, with detailed instructions to record routine operations, processes and practices followed. This template has been freely provided by. Web clickup's quantitative research sop template is designed to help you streamline your quantitative research processes and ensure consistency across your team. Web in clinical research, sops help define the group’s (e.g., unit, division, department, institution, etc.) standard practices and daily processes conducted to assure execution. The standard operating procedures (sops) in this library are established to ensure consistency and compliance with federal/state. List of standard operating procedures. Via the global health network. Web this standard operating procedure (sop) describes the standard format and method the uh clinical research center (crc) policy oversight committee will use inwriting and. Web clinical study report template. Occupational and environmental safety office (oeso) financial services. [title of the sop] [insert clinical research site (crs) name and crs number] [insert. Budget monitoring tool with example data. Web in clinical research, sops help define the group’s (e.g., unit, division, department, institution, etc.) standard practices and daily processes conducted to assure. Web institutional policies and procedures. Follow these six steps to create quality sops that meet ethical and regulatory standards. Occupational and environmental safety office (oeso) financial services. Web appendix standard operating procedure template. Web what are standard operating procedures (sop)? Web clinical research standard operating procedures. Web clinical study report template. An sop is the principal document, which describes what is to be done, who is responsible. Web in clinical research, sops help define the group’s (e.g., unit, division, department, institution, etc.) standard practices and daily processes conducted to assure. Budget monitoring tool with example data. [title of the sop] [insert clinical research site (crs) name and crs number] [insert. Web standard operating procedures (sops) are uniformly written procedures, with detailed instructions to record routine operations, processes and practices followed. Web learn how to write clear and consistent sops for clinical trials and research. Web clinical research sops. Web community health network office of research administration sops for the conduct of clinical research * templates are optional tools that can be used or revised per. Web below are the standard operating procedures of the clinical research center.Clinical Research sop Template Free Of Ich Gcp E6 R2 Addendum Risk
FREE 35+ SOP Templates in PDF
Clinical Research sop Template Free Of Description Of Pharmacovigilance
Clinical Research sop Template Free Of Sample Crc Resume by Pharma
FREE 61+ SOP Templates in PDF MS Word
Clinical Research sop Template Free Of Microsoft Word sop Recruitment
FREE 45 SOP Templates in PDF MS Word
61 Clinical Research sop Template Free Heritagechristiancollege
SOP TM00902 Clinical Study Report
FREE 45 SOP Templates in PDF MS Word
This Template Has Been Freely Provided By.
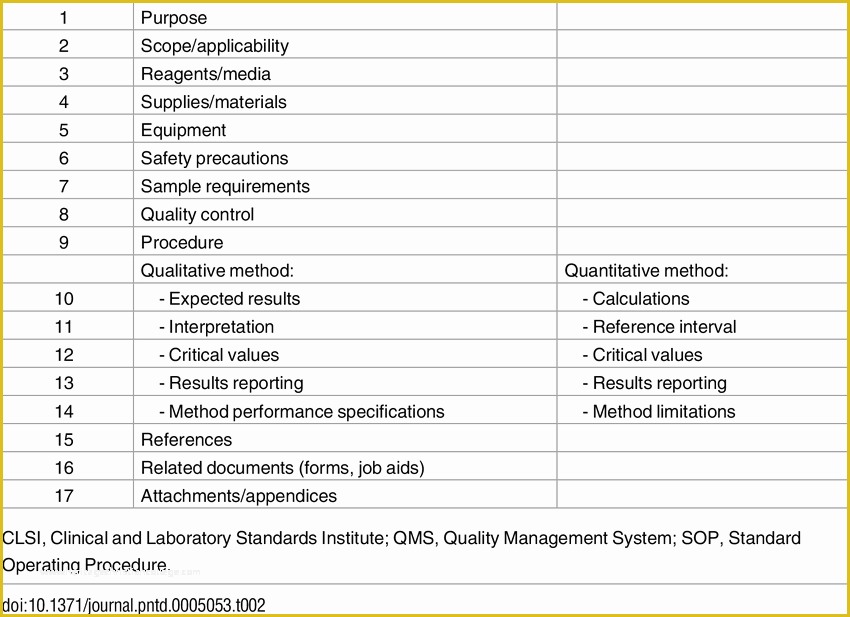
Table Of Contents, Glossary & Abbreviations.
Process For Obtaining Informed Consent 4:
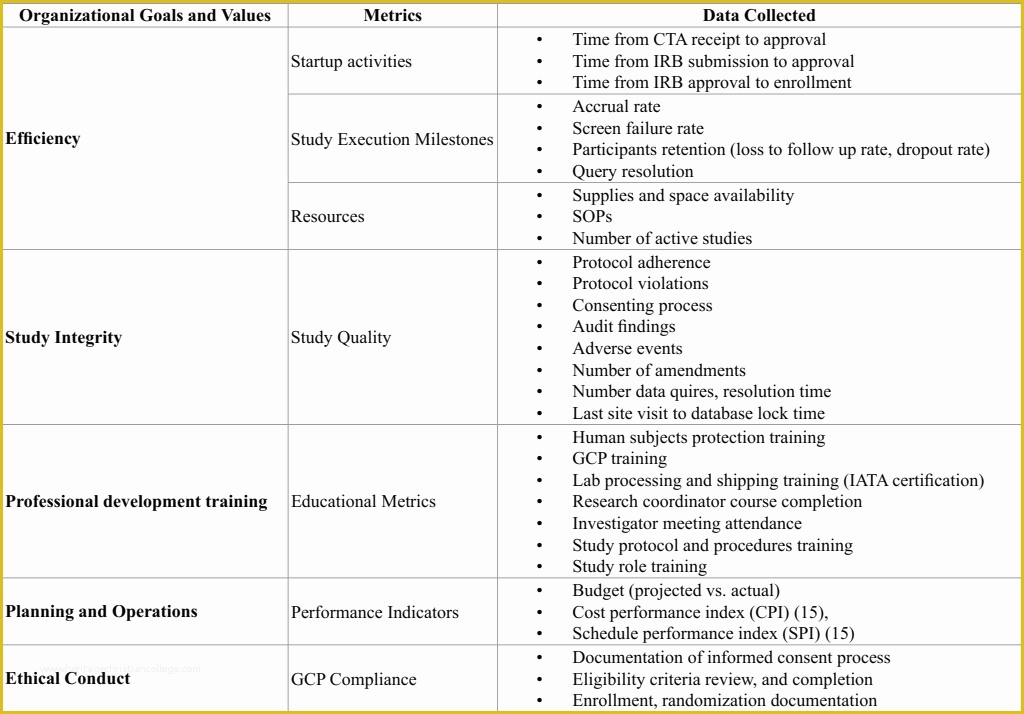
Web Sops Are Available In The Areas Of:
Related Post: