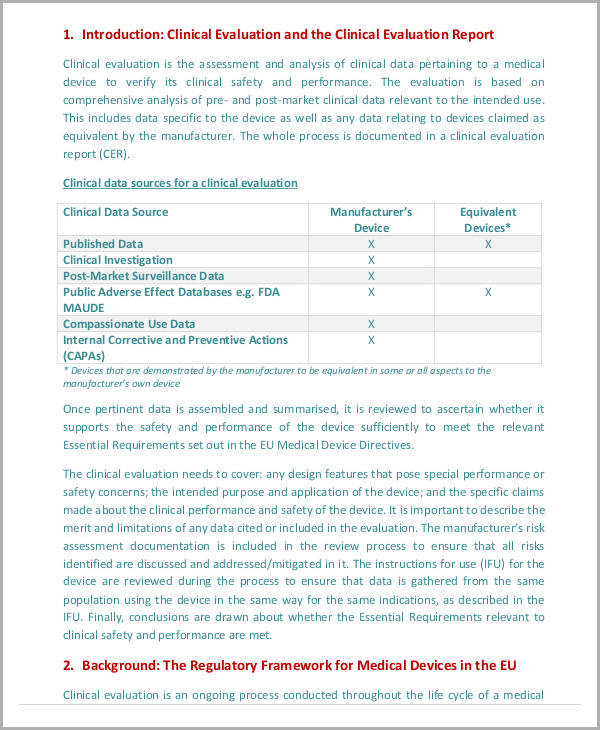
Clinical Evaluation Plan Template
Clinical Evaluation Plan Template - Nb will assess adequacy of clinical investigation plan (cip) to demonstrate “safety, performance and benefit risk of subject devices” 21 ©. Documents include placeholder marks for all. Oliver eidel template download this is a free template,. Possible compensation up to $11,250. Web clinical evaluation assessment report template, specifying recommended minimum content for a notified body clinical evaluation assessment table 1: Ad simplify treatment planning with a fully integrated and customizable template library. Web evaluation plan template {state program name} evaluation plan for {years covered} prepared by: {names} {affiliation} {date} use this template for writing your evaluation. Find the clinical evaluation plan template you require. Web preview clinical evaluation plan template. Web preview clinical evaluation plan template. The document is fully editable so that you can adapt it to your company design. Find local clinical trial opportunities. Ad simplify treatment planning with a fully integrated and customizable template library. Web institute for clinical and translational research (ictr) accelerated translational incubator pilot (atip) grant program. Free, easy returns on millions of items. You can download it as word (.docx), pdf, google docs or markdown file. Oliver eidel template download this is a free template,. Ad simplify treatment planning with a fully integrated and customizable template library. Ad learn how you can qualify for local clinical studies. You can download it as word (.docx), pdf, google docs or markdown file. All medical devices marketed in eu member state countries must undertake. Web clinical evaluation plan <city, state, zip> device 1summary4. Web clinical evaluation assessment report template, specifying recommended minimum content for a notified body clinical evaluation assessment table 1: Web institute for clinical and translational research (ictr). The clinical evaluation procedure explains the entire clinical evaluation process beginning from the scope and plan through the. Learn why we're a preferred mobile research provider for decentralized clinical trials. Our templates currently cover compliance for iso 13485, iec 62304, iso 14971 and iec 62366. You can download it as word (.docx), pdf, google docs or markdown file. Find deals. Nb will assess adequacy of clinical investigation plan (cip) to demonstrate “safety, performance and benefit risk of subject devices” 21 ©. Ad simplify treatment planning with a fully integrated and customizable template library. The clinical evaluation plan, necessary for creating the cer, is detailed in paragraph 1 of part a of annex 14. Free, easy returns on millions of items.. Oliver eidel template download this is a free template,. The clinical evaluation procedure explains the entire clinical evaluation process beginning from the scope and plan through the. Oliver eidel template download this is a free template, provided by openregulatory. Web clinical evaluation plan <city, state, zip> device 1summary4. Our templates currently cover compliance for iso 13485, iec 62304, iso 14971. Learn why we're a preferred mobile research provider for decentralized clinical trials. Web templates clinical evaluation templates updated october 24, 2022 template: Nb will assess adequacy of clinical investigation plan (cip) to demonstrate “safety, performance and benefit risk of subject devices” 21 ©. Web clinical evaluation procedure & templates. Find the clinical evaluation plan template you require. Web clinical evaluation assessment report template, specifying recommended minimum content for a notified body clinical evaluation assessment table 1: Web download them for free and get your compliance done, no strings attached. Web free whitepaper clinical evaluation for medical devices under mdr in this whitepaper, we’ll guide you through crucial regulatory documents pertaining to the. Free, easy returns on millions. Ad free shipping on qualified orders. Oliver eidel template download this is a free template, provided by openregulatory. Learn why we're a preferred mobile research provider for decentralized clinical trials. Open it up using the online editor. Web institute for clinical and translational research (ictr) accelerated translational incubator pilot (atip) grant program. Ad simplify treatment planning with a fully integrated and customizable template library. Documents include placeholder marks for all. All medical devices marketed in eu member state countries must undertake. Clinical studies advance scientific knowledge. Web templates clinical evaluation templates updated october 24, 2022 template: Oliver eidel template download this is a free template,. Ad put the experience of our mobile research nurses to work for your trial. Clinical studies advance scientific knowledge. Web clinical evaluation assessment report template, specifying recommended minimum content for a notified body clinical evaluation assessment table 1: Ad simplify treatment planning with a fully integrated and customizable template library. Web templates clinical evaluation templates updated october 24, 2022 template: Oliver eidel template download this is a free template, provided by openregulatory. Web clinical evaluation procedure & templates. {names} {affiliation} {date} use this template for writing your evaluation. The document is fully editable so that you can adapt it to your company design. Documents include placeholder marks for all. Web preview clinical evaluation plan template. Web clinical evaluation assessment report template july 2020 this document has been endorsed by the medical device coordination group (mdcg) established by article 103. You can download it as word (.docx), pdf, google docs or markdown file. Ad simplify treatment planning with a fully integrated and customizable template library. Web the clinical evaluation plan defines methods for creating and updating the clinical evaluation report. Web free whitepaper clinical evaluation for medical devices under mdr in this whitepaper, we’ll guide you through crucial regulatory documents pertaining to the. Nb will assess adequacy of clinical investigation plan (cip) to demonstrate “safety, performance and benefit risk of subject devices” 21 ©. Web institute for clinical and translational research (ictr) accelerated translational incubator pilot (atip) grant program. Ad learn how you can qualify for local clinical studies. Oliver eidel template download this is a free template, provided by openregulatory. All medical devices marketed in eu member state countries must undertake. Nb will assess adequacy of clinical investigation plan (cip) to demonstrate “safety, performance and benefit risk of subject devices” 21 ©. Find local clinical trial opportunities. The clinical evaluation plan, necessary for creating the cer, is detailed in paragraph 1 of part a of annex 14. Web what is a clinical evaluation plan? Ad simplify treatment planning with a fully integrated and customizable template library. Find the clinical evaluation plan template you require. Find deals and low prices on popular products at amazon.com Web free whitepaper clinical evaluation for medical devices under mdr in this whitepaper, we’ll guide you through crucial regulatory documents pertaining to the. You can download it as word (.docx), pdf, google docs or markdown file. Free, easy returns on millions of items. Ad put the experience of our mobile research nurses to work for your trial. Web clinical evaluation assessment report template, specifying recommended minimum content for a notified body clinical evaluation assessment table 1: The clinical evaluation procedure explains the entire clinical evaluation process beginning from the scope and plan through the. Documents include placeholder marks for all.final clinical evaluation Nursing Learning
Clinical Evaluation Procedure Bundle
Template For Evaluation Report
Product Evaluation Template Free Word and Excel Templates
Clinical Evaluation Procedure
FREE 8+ Evaluation Plan Templates in MS Word PDF
Clinical Evaluation Procedure Bundle
Clinical Evaluation Plan/Report Fill and Sign Printable Template
Download Clinical Skills Assessment Template for Free Page 2
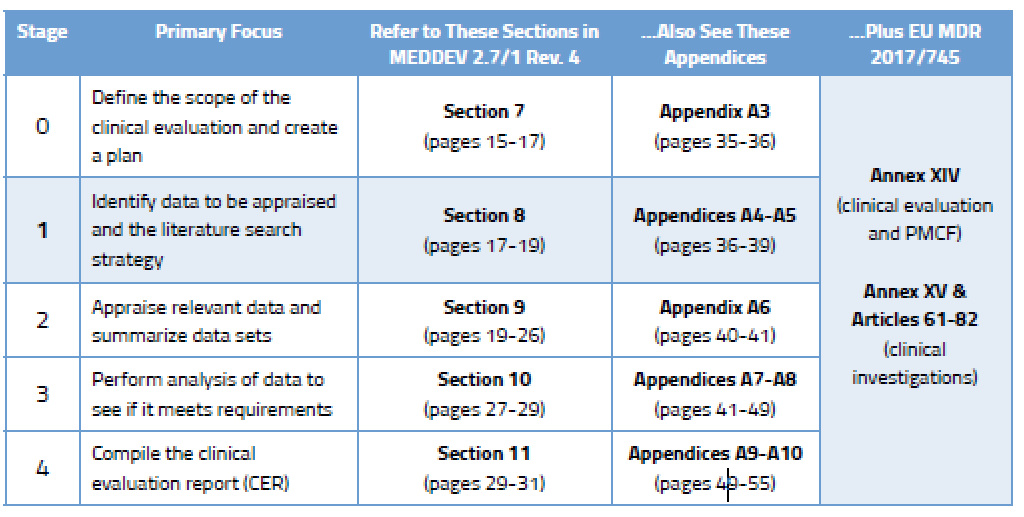
Creating an EU CER Literature Review Protocol for Medical Devices
{Names} {Affiliation} {Date} Use This Template For Writing Your Evaluation.
Ad Free Shipping On Qualified Orders.
The Document Is Fully Editable So That You Can Adapt It To Your Company Design.
Web Clinical Evaluation Assessment Report Template July 2020 This Document Has Been Endorsed By The Medical Device Coordination Group (Mdcg) Established By Article 103.
Related Post: