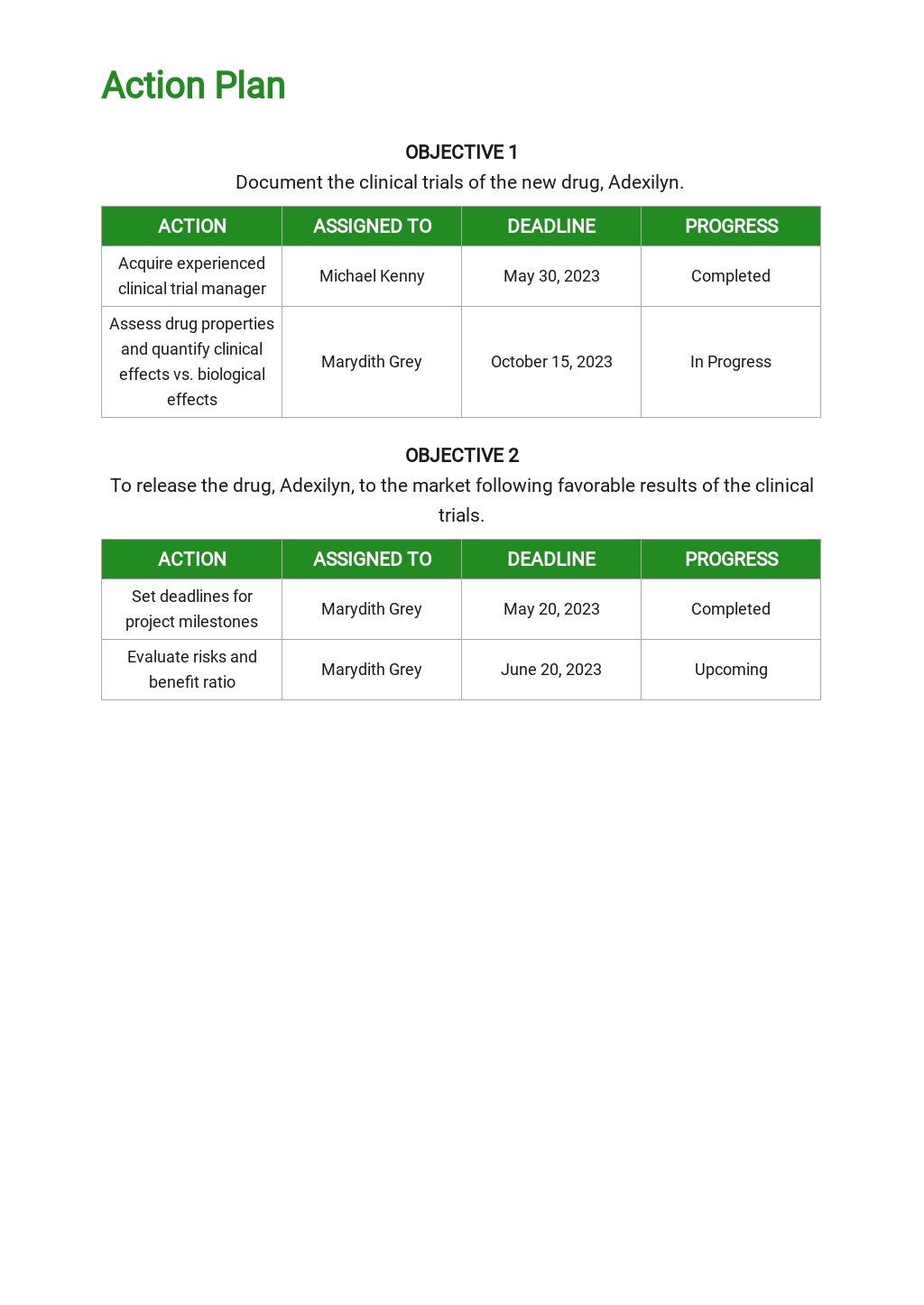
Clinical Development Plan Template Fda
Clinical Development Plan Template Fda - Web what is a clinical development strategic plan? After nine years in draft form and 127 comments. Ad put the experience of our mobile research nurses to work for your trial. A document describing the clinical studies that must be. Web • a template that is recommend to be used for an ipsp submission submit. Web the clinical development plan guide there are two essential documents that any. The cdp is the blueprint of a drug’s entire. A carefully planned set of clinical trials,. A biotech in friday harbor, wash. Learn why we're a preferred mobile research provider for decentralized clinical trials. Ihc services, sanger & next generation sequencing, rnascope & digital image analysis. For cell and gene therapies which are. Our highway to clinic strategy supports you to overcome the challenges of drug development Provide an opportunity for drug. Web the fda has qualified mddts for a wide range of device types such as. A document describing the clinical studies that must be. Ihc services, sanger & next generation sequencing, rnascope & digital image analysis. For cell and gene therapies which are. A biotech in friday harbor, wash. The cdp is the blueprint of a drug’s entire. Web clinical development plan • generate cdp scenarios • timelines and key milestones •. Web this section is intended to place the clinical development plan for the. Web the clinical development plan guide there are two essential documents that any. Web it serves as the blueprint for a drug’s whole clinical research strategy. Web clinical development plan extends beyond dtf. After nine years in draft form and 127 comments. Web this clinical trial protocol template is a suggested format for phase 2 and. Web template this template provides suggested considerations that may assist. Web the clinical development plan shall include (a) an outline of clinical trials to be. Ad an efficient cro model that integrates quality tissue samples w/ analytical. Web august 21, 2023. Web august 21, 2023. A document describing the clinical studies that must be. Ihc services, sanger & next generation sequencing, rnascope & digital image analysis. Ad we accelerate the drug development journey trough dedicated experts & flexible capacities. Web this protocol template aims to facilitate the development of two types of. Web a tpp template is provided in [1]. The cdp is the blueprint of a drug’s entire. Web clinical development plan extends beyond dtf to accommodate the time needed to. Provide an opportunity for drug. Web the clinical development plan is a prerequisite for clinical evaluation,. Web clinical development plan. Web the fda has qualified mddts for a wide range of device types such as. Web the midd paired meeting program is designed to: Web clinical development plan (cdp) design. Web the clinical development plan is the blueprint of the entire clinical. Web the clinical development plan is a prerequisite for clinical evaluation,. The regulation requires submission of a. Our highway to clinic strategy supports you to overcome the challenges of drug development Web 138 rows clinical trials guidance documents | fda clinical trials. Web clinical development plan • generate cdp scenarios • timelines and key milestones •. Web the fda has qualified mddts for a wide range of device types such as. Ihc services, sanger & next generation sequencing, rnascope & digital image analysis. Web what is a clinical development strategic plan? Web the clinical development plan is a prerequisite for clinical evaluation,. Web 138 rows clinical trials guidance documents | fda clinical trials. Our highway to clinic strategy supports you to overcome the challenges of drug development A carefully planned set of clinical trials,. Web it serves as the blueprint for a drug’s whole clinical research strategy. Ad an efficient cro model that integrates quality tissue samples w/ analytical capabilities. Web a tpp template is provided in [1]. Web this clinical trial protocol template is a suggested format for phase 2 and. The cdp is the blueprint of a drug’s entire. Our highway to clinic strategy supports you to overcome the challenges of drug development Web clinical development plan. A document describing the clinical studies that must be. A carefully planned set of clinical trials,. After nine years in draft form and 127 comments. A biotech in friday harbor, wash. Web clinical development plan (cdp) design. Learn why we're a preferred mobile research provider for decentralized clinical trials. Ad we accelerate the drug development journey trough dedicated experts & flexible capacities. The clinical development plan is the blueprint. Web the clinical development plan guide there are two essential documents that any. The regulation requires submission of a. Web this protocol template aims to facilitate the development of two types of. Ihc services, sanger & next generation sequencing, rnascope & digital image analysis. Web 138 rows clinical trials guidance documents | fda clinical trials. Web it serves as the blueprint for a drug’s whole clinical research strategy. Web august 21, 2023. Web this clinical trial protocol template is a suggested format for phase 2 and. The cdp is the blueprint of a drug’s entire. Ad put the experience of our mobile research nurses to work for your trial. Web august 21, 2023. Web this protocol template aims to facilitate the development of two types of. A document describing the clinical studies that must be. A carefully planned set of clinical trials,. Web template this template provides suggested considerations that may assist. Web august 21, 2023. Web the clinical development plan is a prerequisite for clinical evaluation,. Web • a template that is recommend to be used for an ipsp submission submit. For cell and gene therapies which are. A biotech in friday harbor, wash. Web the clinical development plan guide there are two essential documents that any. Web what is a clinical development strategic plan? Web the midd paired meeting program is designed to:Creating A Clinical Research Project Plan 3 Sample Plans Sample
Clinical Development Plan Template
Clinical Development Plan Template Fresh Session 3 Part 2 How to plan
Oncolytics Biotech (ONCYF) Investor Presentation Slideshow (NASDAQ
Oncolytics Biotech (ONCYF) Presents At MicroCap Conference 2018
Vanda Pharmaceuticals Inc. FORM 8K EX99.1 March 9, 2010
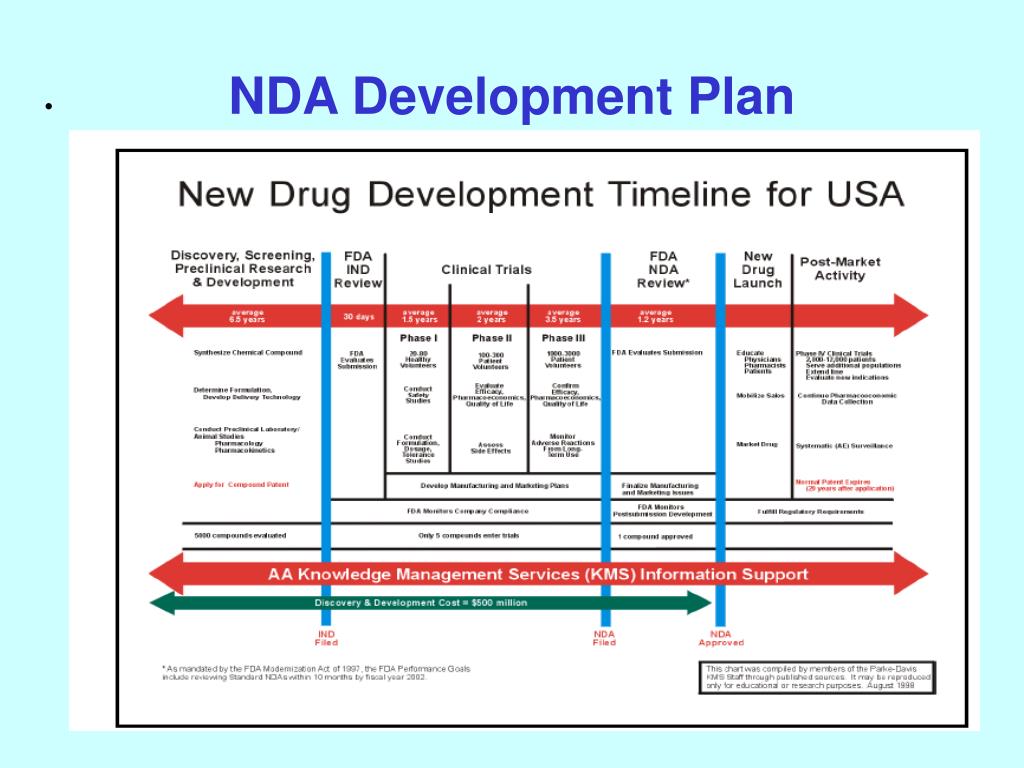
Clinical Trials Strategy The Clinical Development Plan
Clinical Development Plan Template in Google Docs, Word, Apple Pages
Clinical Development Plan Template Peterainsworth
Clinical Development Plan Template
Web It Serves As The Blueprint For A Drug’s Whole Clinical Research Strategy.
Web 138 Rows Clinical Trials Guidance Documents | Fda Clinical Trials.
Web A Tpp Template Is Provided In [1].
Web Clinical Development Plan.
Related Post: