Capa Template Clinical Research
Capa Template Clinical Research - Guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying deviation patterns and areas of. Web capas can be used for audit or inspection observations, compliance improvement, or risk mitigation. Web clinical capa process, managing clinical quality using corrective and preventative actions is not new to clinical. Web corrective and preventive actions (capa) plans. The first step in developing a capa plan is to identify. Web corrective action and preventive action (capa) plan template. Understand what findings require an action plan vs. Web corrective and preventive actions (capa) inspectional objectives. Web learn about capa — corrective actions and preventive actions — and how its processes seek out and sustain safety and quality across multiple industries. Corrective and preven tative action (capa) p lan last revised:. Web corrective action and preventive action (capa) plan template. Easy to use, instant deployment. The first step in developing a capa plan is to identify. Identify common findings found in research study reviews conducted by the ctqa program. The international conference for harmonization (ich) released. Web capa is written to identify a discrepancy/problem in the conduct of a clinical research study, note the root cause of the identified problem, identify the corrective action to. Download corrective and preventative action plan form template_2019.11.13. Web corrective action and preventive action (capa) plan template. Discuss all research procedures involved in the human research for each subject population participating. Identify common findings found in research study reviews conducted by the ctqa program. Web standard operating procedures (sop) for clinical research title: Web corrective and preventive actions (capa) plans. Track and manage quality events. Web five best practices. Discuss all research procedures involved in the human research for each subject population participating in the study. Web capas in clinical research fda guidance: Web in this course, participants will learn the process for developing an effective corrective and preventive action plan (capa). Web five best practices. Web corrective and preventive actions (capa) plans. Web capas in clinical research fda guidance: •your capa might have to involve other studies under the pi and/or using the same study staff. The international conference for harmonization (ich) released. Web the iu hrpp quality improvement office (qio) has developed a capa plan template to assist study teams with this activity. Corrective and preven tative action (capa) p lan. Web learn about capa — corrective actions and preventive actions — and how its processes seek out and sustain safety and quality across multiple industries. Guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying deviation patterns and areas of. Web corrective action and preventive action (capa) plan template. Download corrective and preventative action plan form template_2019.11.13. Web the. Web this article provides an overview of the root cause of these problems and how to ensure that corrective and preventive actions are addressing the actual problem. Web the iu hrpp quality improvement office (qio) has developed a capa plan template to assist study teams with this activity. Web five best practices. Verify that capa system procedure(s) that address the. Web capas in clinical research fda guidance: The fda indicates that corrective and preventive actions (capas) are absolutely necessary to resolve problems and. Web capa is written to identify a discrepancy/problem in the conduct of a clinical research study, note the root cause of the identified problem, identify the corrective action to. Discuss all research procedures involved in the human. Web include how long subject participation is expected to last. Identify common findings found in research study reviews conducted by the ctqa program. It will cover when and why capas are. Web clinical research is the catalyst for bringing groundbreaking scientific discoveries to the forefront in finding new ways to treat, prevent and detect illnesses. Web the iu hrpp quality. Let us take a look at five capa best practices that can strengthen clinical research compliance and also help accomplish a fundamental goal. Web clinical capa process, managing clinical quality using corrective and preventative actions is not new to clinical. Identify common findings found in research study reviews conducted by the ctqa program. Corrective and preventive actions (capa) plans. Understand. The first step in developing a capa plan is to identify. Guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying deviation patterns and areas of. Easy to use, instant deployment. Web corrective and preventive actions (capa) plans. Discuss all research procedures involved in the human research for each subject population participating in the study. Web corrective and preventive actions (capa) inspectional objectives. •your capa might have to involve other studies under the pi and/or using the same study staff. Web in this course, participants will learn the process for developing an effective corrective and preventive action plan (capa). Let us take a look at five capa best practices that can strengthen clinical research compliance and also help accomplish a fundamental goal. Web clinical research is the catalyst for bringing groundbreaking scientific discoveries to the forefront in finding new ways to treat, prevent and detect illnesses. Download corrective and preventative action plan form template_2019.11.13. Web capa is written to identify a discrepancy/problem in the conduct of a clinical research study, note the root cause of the identified problem, identify the corrective action to. Web five best practices. Web capas in clinical research fda guidance: Web learn about capa — corrective actions and preventive actions — and how its processes seek out and sustain safety and quality across multiple industries. Guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying. A form has been created to facilitate the capa (corrective action /. Web capas can be used for audit or inspection observations, compliance improvement, or risk mitigation. The international conference for harmonization (ich) released. Web the iu hrpp quality improvement office (qio) has developed a capa plan template to assist study teams with this activity. Web standard operating procedures (sop) for clinical research title: The fda indicates that corrective and preventive actions (capas) are absolutely necessary to resolve problems and. Discuss all research procedures involved in the human research for each subject population participating in the study. •your capa might have to involve other studies under the pi and/or using the same study staff. Web clinical research is the catalyst for bringing groundbreaking scientific discoveries to the forefront in finding new ways to treat, prevent and detect illnesses. Understand what findings require an action plan vs. Guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying deviation patterns and areas of. Web five best practices. Web include how long subject participation is expected to last. Corrective and preven tative action (capa) p lan last revised:. Web in this course, participants will learn the process for developing an effective corrective and preventive action plan (capa). The international conference for harmonization (ich) released. Download corrective and preventative action plan form template_2019.11.13. Web learn about capa — corrective actions and preventive actions — and how its processes seek out and sustain safety and quality across multiple industries. Web this article provides an overview of the root cause of these problems and how to ensure that corrective and preventive actions are addressing the actual problem. Web capas in clinical research fda guidance:Capa Report Template Luxury 8d Report On Capa Item in 2020 Report
Capa Mock Sop Clinical Trial Medicine
Sample Capa form Peterainsworth
Howtocreate capa template
A Free CAPA Template for the Medical Device Industry
PPT Ensuring Quality in Medical Device Clinical Trials PowerPoint
15+ Capa Vorlage MelTemplates MelTemplates
CAPA form Corrective action and preventive action
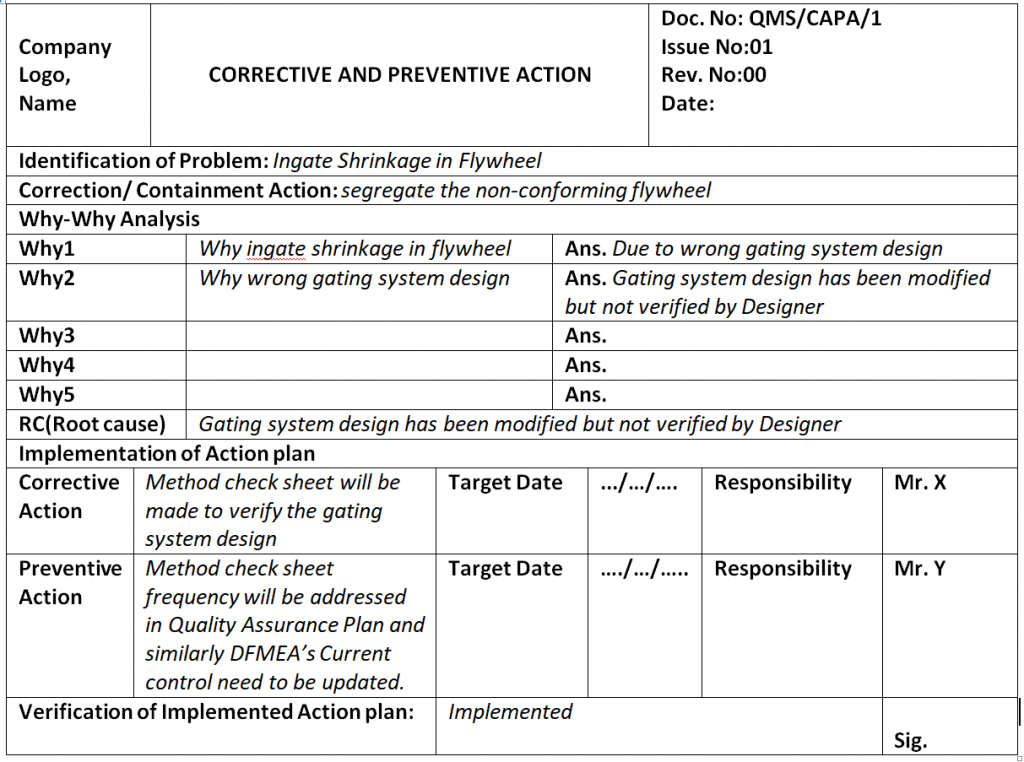
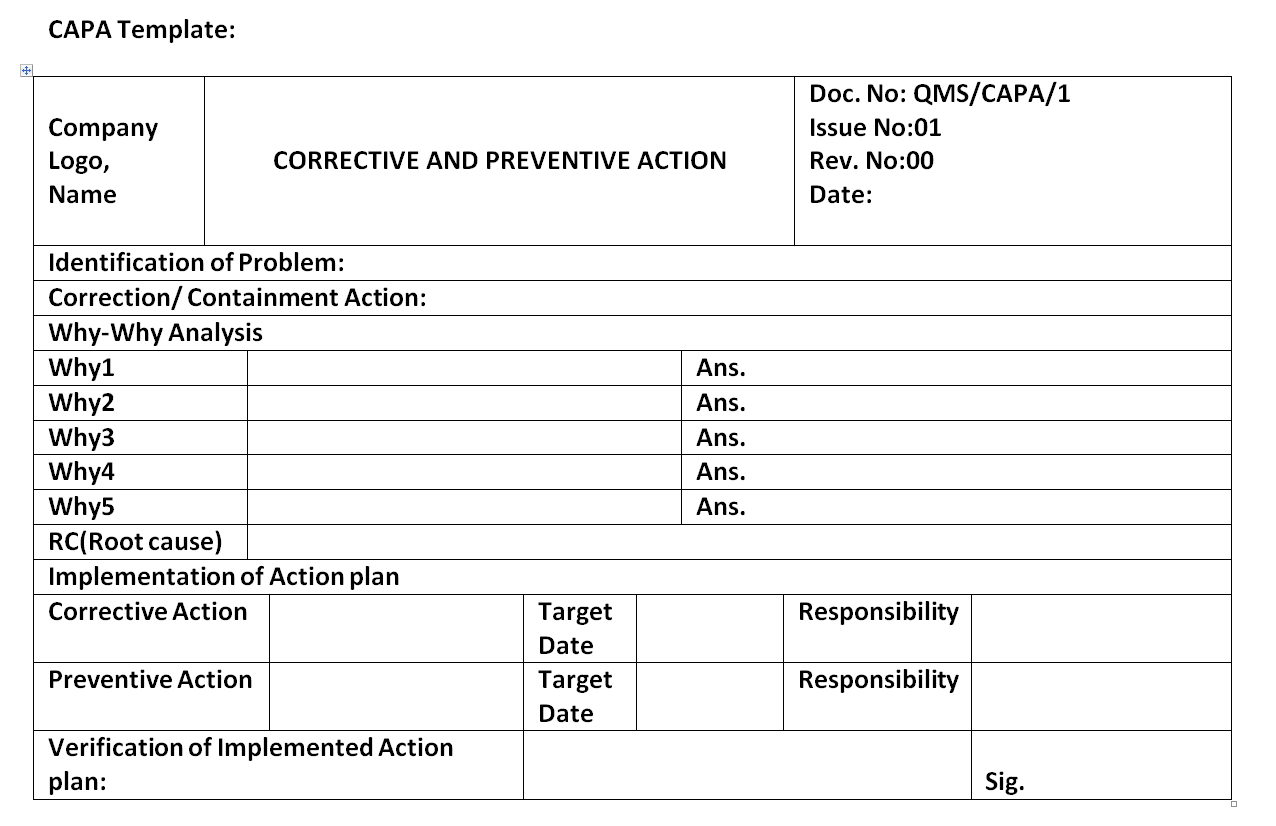
Corrective and Preventive Action Format CAPA with Example Download
Clinical Trial Report Template (1) TEMPLATES EXAMPLE TEMPLATES
The First Step In Developing A Capa Plan Is To Identify.
Let Us Take A Look At Five Capa Best Practices That Can Strengthen Clinical Research Compliance And Also Help Accomplish A Fundamental Goal.
Web Capas Can Be Used For Audit Or Inspection Observations, Compliance Improvement, Or Risk Mitigation.
This Template Is Designed To.
Related Post: